Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel
- PMID: 20501650
- PMCID: PMC2906337
- DOI: 10.1074/jbc.M109.093500
Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel
Abstract
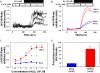
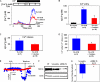
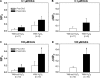
This study examined the effect of H(2)O(2) on the TRPC6 channel and its underlying mechanisms using a TRPC6 heterologous expression system. In TRPC6-expressing HEK293T cells, H(2)O(2) significantly stimulated Ca(2+) entry in a dose-dependent manner. Electrophysiological experiments showed that H(2)O(2) significantly increased TRPC6 channel open probability and whole-cell currents. H(2)O(2) also evoked a robust inward current in A7r5 vascular smooth muscle cells, which was nearly abolished by knockdown of TRPC6 using a small interfering RNA. Catalase substantially attenuated arginine vasopressin (AVP)-induced Ca(2+) entry in cells co-transfected with TRPC6 and AVP V1 receptor. N-Ethylmaleimide and thimerosal were able to simulate the H(2)O(2) response. Dithiothreitol or glutathione-reduced ethyl ester significantly antagonized the response. Furthermore, both N-ethylmaleimide- and H(2)O(2)-induced TRPC6 activations were only observed in the cell-attached patches but not in the inside-out patches. Moreover, 1-oleoyl-2-acetyl-sn-glycerol effect on TRPC6 was significantly greater in the presence of H(2)O(2). Biotinylation assays revealed a significant increase in cell surface TRPC6 in response to H(2)O(2). Similarly, in cells transfected with TRPC6-EGFP, confocal microscopy showed a significant increase in fluorescence intensity in the region of the cell membrane and adjacent to the membrane. AVP also increased the fluorescence intensity on the surface of the cells co-transfected with TRPC6-EGFP and V1 receptor, and this response was inhibited by catalase. These data indicate that H(2)O(2) activates TRPC6 channels via modification of thiol groups of intracellular proteins. This cysteine oxidation-dependent pathway not only stimulates the TRPC6 channel by itself but also sensitizes the channels to diacylglycerol and promotes TRPC6 trafficking to the cell surface.
Figures


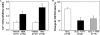


References
-
- Lassègue B., Griendling K. K. (2004) Am. J. Hypertens. 17, 852–860 - PubMed
-
- Rhee S. G., Chang T. S., Bae Y. S., Lee S. R., Kang S. W. (2003) J. Am. Soc. Nephrol. 14, S211–S215 - PubMed
-
- Hidalgo C., Donoso P. (2008) Antioxid. Redox. Signal. 10, 1275–1312 - PubMed
-
- Schnackenberg C. G. (2002) Curr. Opin. Pharmacol. 2, 121–125 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

