Impaired-inactivation of FoxO1 contributes to glucose-mediated increases in serum very low-density lipoprotein
- PMID: 20501667
- PMCID: PMC2940519
- DOI: 10.1210/en.2010-0204
Impaired-inactivation of FoxO1 contributes to glucose-mediated increases in serum very low-density lipoprotein
Abstract
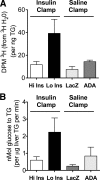
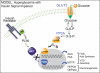
For patients with diabetes, insulin resistance and hyperglycemia both contribute to increased serum triglyceride in the form of very low-density lipoprotein (VLDL). Our objective was to define the insulin conditions in which hyperglycemia promotes increased serum VLDL in vivo. We performed hyperglycemic-hyperinsulinemic clamp studies and hyperglycemic-hypoinsulinemic clamp studies in rats, with metabolic tracers for glucose flux and de novo fatty acid synthesis. When blood glucose was clamped at hyperglycemia (17 mm) for 2 h under hyperinsulinemic conditions (4 mU/kg . min), serum VLDL levels were not increased compared with baseline. We speculated that hyperinsulinemia minimized glucose-mediated VLDL changes and performed hyperglycemic-hypoinsulinemic clamp studies in which insulin was clamped near fasting levels with somatostatin (17 mm blood glucose, 0.25 mU/kg . min insulin). Under low-insulin conditions, serum VLDL levels were increased 4.7-fold after hyperglycemia, and forkhead box O1 (FoxO1) was not excluded from the nucleus of liver cells. We tested the extent that impaired inactivation of FoxO1 by insulin was sufficient for glucose to promote increased serum VLDL. We found that, when the ability of insulin to inactivate FoxO1 is blocked after adenoviral delivery of constitutively active FoxO1, glucose increased serum VLDL triglyceride when given both by ip glucose tolerance testing (3.5-fold increase) and by a hyperglycemic clamp (4.6-fold). Under both experimental conditions in which insulin signaling to FoxO1 was impaired, we found increased activation of carbohydrate response element binding protein. These data suggest that glucose more potently promotes increased serum VLDL when insulin action is impaired, with either low insulin levels or disrupted downstream signaling to the transcription factor FoxO1.
Figures


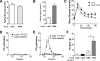


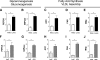
References
-
- Adiels M, Borén J, Caslake MJ, Stewart P, Soro A, Westerbacka J, Wennberg B, Olofsson SO, Packard C, Taskinen MR 2005 Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 25:1697–1703 - PubMed
-
- Bernstein RM, Davis BM, Olefsky JM, Reaven GM 1978 Hepatic insulin responsiveness in patients with endogenous hypertriglyceridaemia. Diabetologia 14:249–253 - PubMed
-
- Gill JM, Brown JC, Bedford D, Wright DM, Cooney J, Hughes DA, Packard CJ, Caslake MJ 2004 Hepatic production of VLDL1 but not VLDL2 is related to insulin resistance in normoglycaemic middle-aged subjects. Atherosclerosis 176:49–56 - PubMed
-
- Kissebah AH, Alfarsi S, Adams PW, Wynn V 1976 Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 12:563–571 - PubMed
-
- Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP 1992 Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 41:715–722 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

