Successful treatment of Castleman's disease with interleukin-1 receptor antagonist (Anakinra)
- PMID: 20501803
- PMCID: PMC3108331
- DOI: 10.1158/1535-7163.MCT-10-0156
Successful treatment of Castleman's disease with interleukin-1 receptor antagonist (Anakinra)
Abstract
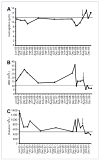
Castleman's disease (CD) is a very rare lymphoproliferative disorder whose underlying pathophysiology is not fully understood and for which no standard treatment exists. Because interleukin-1 (IL-1) might promote the production of interleukin-6 (IL-6), a key pathogenic factor for the disease, we hypothesized that blocking the interleukin-1 receptor would be a useful therapy for CD. We report the case of a 61-year-old woman with CD who had undergone multiple treatments, including cladribine, rituximab, steroids, etanercept, and anti-IL-6 monoclonal antibody, and whose disease was refractory to all of these treatments. She was started on the recombinant IL-1 receptor antagonist, Anakinra, at a subcutaneous dose of 100 mg daily. Within one week, her fatigue and anorexia markedly improved, and her laboratory abnormalities, including anemia, thrombocytosis, leukocytosis, and elevated markers of inflammation, all resolved. Our observation suggests that Anakinra may be an attractive therapeutic approach for refractory multicentric CD.
Conflict of interest statement
R. Kurzrock: Commercial research support and honoraria, AMGEN. No other potential conflicts of interest were disclosed.
Figures
References
-
- Dham A, Peterson B. Castleman disease. Curr Opin Hematol. 2007;14:354–9. - PubMed
-
- Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129:3–17. - PubMed
-
- Larroche C, Cacoub P, Godeau P. La maladie de Castleman. Rev Med Interne. 1996;17:1003–13. [article in French] - PubMed
-
- Casper C, Nichols WG, Huang ML, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004;103:1632–4. - PubMed
-
- Katsume A, Saito H, Yamada Y, et al. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman’s disease symptoms emerged in IL-6 transgenic mice. Cytokine. 2002;20:304–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

