Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine
- PMID: 20501829
- PMCID: PMC3171699
- DOI: 10.1158/0008-5472.CAN-10-0563
Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine
Abstract
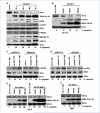
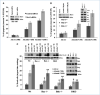
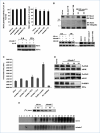
Melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24), a cytokine belonging to the IL-10 family, selectively induces apoptosis in cancer cells without harming normal cells by promoting an endoplasmic reticulum (ER) stress response. The precise molecular mechanism by which the ER stress response culminates in cell death requires further clarification. The present study shows that in prostate carcinoma cells, the mda-7/IL-24-induced ER stress response causes apoptosis by translational inhibition of the antiapoptotic protein myeloid cell leukemia-1 (Mcl-1). Forced expression of Mcl-1 blocked mda-7/IL-24 lethality, whereas RNA interference or gene knockout of Mcl-1 markedly sensitized transformed cells to mda-7/IL-24. Mcl-1 downregulation by mda-7/IL-24 relieved its association with the proapoptotic protein Bak, causing oligomerization of Bak and leading to cell death. These observations show the profound role of the Bcl-2 protein family member Mcl-1 in regulating cancer-specific apoptosis induced by this cytokine. Thus, our studies provide further insights into the molecular mechanism of ER stress-induced cancer-selective apoptosis by mda-7/IL-24. As Mcl-1 is overexpressed in the majority of prostate cancers, mda-7/IL-24 might provide an effective therapeutic for this disease.
Figures




References
-
- Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–21. - PubMed
-
- Mabjeesh NJ, Zhong H, Simons JW. Gene therapy of prostate cancer: current and future directions. Endocr Relat Cancer. 2002;9:115–39. - PubMed
-
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–86. - PubMed
-
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

