PTPL1/PTPN13 regulates breast cancer cell aggressiveness through direct inactivation of Src kinase
- PMID: 20501847
- PMCID: PMC3132424
- DOI: 10.1158/0008-5472.CAN-09-4368
PTPL1/PTPN13 regulates breast cancer cell aggressiveness through direct inactivation of Src kinase
Abstract
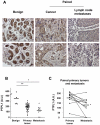
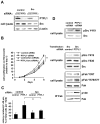
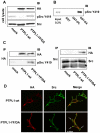
The protein tyrosine phosphatase PTPL1/PTPN13, the activity of which is decreased through allelic loss, promoter methylation, or somatic mutations in some tumors, has been proposed as a tumor suppressor gene. Moreover, our recent clinical study identified PTPL1 expression level as an independent prognostic indicator of a favorable outcome for patients with breast cancer. However, how PTPL1 can affect tumor aggressiveness has not been characterized. Here, we first show that PTPL1 expression, assessed by immunohistochemistry, is decreased in breast cancer and metastasis specimens compared with nonmalignant tissues. Second, to evaluate whether PTPL1 plays a critical role in breast cancer progression, RNA interference experiments were performed in poorly tumorigenic MCF-7 breast cancer cells. PTPL1 inhibition drastically increased tumor growth in athymic mice and also enhanced several parameters associated with tumor progression, including cell proliferation on extracellular matrix components and cell invasion. Furthermore, the inhibition of Src kinase expression drastically blocked the effects of PTPL1 silencing on cell growth. In PTPL1 knockdown cells, the phosphorylation of Src on tyrosine 419 is increased, leading to the activation of its downstream substrates Fak and p130cas. Finally, substrate-trapping experiments revealed that Src tyrosine 419 is a direct target of the phosphatase. Thus, by identification of PTPL1 as the first phosphatase able to inhibit Src through direct dephosphorylation in intact cells, we presently describe a new mechanism by which PTPL1 inhibits breast tumor aggressiveness.
Figures



Similar articles
-
The putative tumor suppressor gene PTPN13/PTPL1 induces apoptosis through insulin receptor substrate-1 dephosphorylation.Cancer Res. 2007 Jul 15;67(14):6806-13. doi: 10.1158/0008-5472.CAN-07-0513. Cancer Res. 2007. PMID: 17638892
-
ErbB2, EphrinB1, Src kinase and PTPN13 signaling complex regulates MAP kinase signaling in human cancers.PLoS One. 2012;7(1):e30447. doi: 10.1371/journal.pone.0030447. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279592 Free PMC article.
-
PTPN13/PTPL1: an important regulator of tumor aggressiveness.Anticancer Agents Med Chem. 2011 Jan;11(1):78-88. doi: 10.2174/187152011794941262. Anticancer Agents Med Chem. 2011. PMID: 21235435 Free PMC article. Review.
-
Expression of the putative tumor suppressor gene PTPN13/PTPL1 is an independent prognostic marker for overall survival in breast cancer.Int J Cancer. 2009 Feb 1;124(3):638-43. doi: 10.1002/ijc.23989. Int J Cancer. 2009. PMID: 19004008 Free PMC article.
-
[Expression and Significance of PTPL1 in Hematological Malignancies].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014 Dec;22(6):1744-7. doi: 10.7534/j.issn.1009-2137.2014.06.045. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014. PMID: 25543508 Review. Chinese.
Cited by
-
The HER2 Signaling Network in Breast Cancer--Like a Spider in its Web.J Mammary Gland Biol Neoplasia. 2014 Dec;19(3-4):253-70. doi: 10.1007/s10911-014-9329-5. Epub 2014 Dec 28. J Mammary Gland Biol Neoplasia. 2014. PMID: 25544707 Review.
-
PTPN3 suppresses lung cancer cell invasiveness by counteracting Src-mediated DAAM1 activation and actin polymerization.Oncogene. 2019 Oct;38(44):7002-7016. doi: 10.1038/s41388-019-0948-6. Epub 2019 Aug 12. Oncogene. 2019. PMID: 31406243
-
EphrinB1: novel microtubule associated protein whose expression affects taxane sensitivity.Oncotarget. 2015 Jan 20;6(2):953-68. doi: 10.18632/oncotarget.2823. Oncotarget. 2015. PMID: 25436983 Free PMC article.
-
Protein Tyrosine Phosphatase Receptor Type G (PTPRG) Controls Fibroblast Growth Factor Receptor (FGFR) 1 Activity and Influences Sensitivity to FGFR Kinase Inhibitors.Mol Cell Proteomics. 2018 May;17(5):850-870. doi: 10.1074/mcp.RA117.000538. Epub 2018 Jan 25. Mol Cell Proteomics. 2018. PMID: 29371290 Free PMC article.
-
A database of breast oncogenic specific siRNAs.Sci Rep. 2017 Aug 18;7(1):8706. doi: 10.1038/s41598-017-08948-1. Sci Rep. 2017. PMID: 28821760 Free PMC article.
References
-
- Bompard G, Martin M, Roy C, Vignon F, Freiss G. Membrane targeting of protein tyrosine phosphatase PTPL1 through its FERM domain via binding to phosphatidylinositol 4,5-biphosphate. J Cell Sci. 2003;116:2519–30. - PubMed
-
- Nakahira M, Tanaka T, Robson BE, Mizgerd JP, Grusby MJ. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity. 2007;26:163–76. - PubMed
-
- Wansink DG, Peters W, Schaafsma I, et al. Mild impairment of motor nerve repair in mice lacking PTP-BL tyrosine phosphatase activity. Physiol Genomics. 2004;19:50–60. - PubMed
-
- Glondu-Lassis M, Dromard M, Chavey C, et al. Downregulation of protein tyrosine phosphatase PTP-BL represses adipogenesis. Int J Biochem Cell Biol. 2009;41:2173–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

