Tetraspanin CD151 regulates growth of mammary epithelial cells in three-dimensional extracellular matrix: implication for mammary ductal carcinoma in situ
- PMID: 20501858
- PMCID: PMC2883732
- DOI: 10.1158/0008-5472.CAN-09-4330
Tetraspanin CD151 regulates growth of mammary epithelial cells in three-dimensional extracellular matrix: implication for mammary ductal carcinoma in situ
Abstract
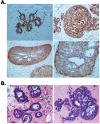
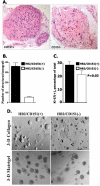
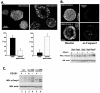
Tetraspanin CD151 is associated with laminin-binding integrins (i.e., alpha(3)beta(1), alpha(6)beta(1), and alpha(6)beta(4)) and regulates tumor cell migration and invasion. Here, we examined the role of CD151 in proliferation of mammary epithelial cells using in vitro and in vivo models. Depletion of CD151 suppressed growth of HB2 cells, a nontumorigenic breast epithelial cell line, in three-dimensional (3D) extracellular matrices (ECM) and in Matrigel-based xenografts. Whereas the presence of alpha(3)beta(1) (but not alpha(6) integrins) was necessary to support growth of HB2 cells in 3D ECM, the pro-proliferative activity of CD151 did not require direct interaction with integrins. Furthermore, depletion of CD151 potentiated formation of the internal lumen and partial restoration of polarity when HB2 cells were cultured in 3D ECM. This correlated with a decrease in phosphorylation levels of extracellular signal-regulated kinase 1/2 and cAkt in CD151-negative cells and increase in activation of caspase-3. Accordingly, the number of CD151-positive colonies with internal lumen was increased by approximately 5-fold when cells were cultured in the presence of MAP/ERK kinase (U0126) and phosphoinositide 3-kinase (LY29004) inhibitors. To establish the physiologic relevance of pro-proliferative and morphogenetic activities of CD151, we analyzed the expression of this tetraspanin in ductal carcinoma in situ (DCIS), which is characterized by neoplastic proliferation of mammary epithelial cells. Strong homogeneous membrane expression of CD151 was found to be associated with a high grade of DCIS (P = 0.004). Taken together, these results strongly suggest that CD151 complexes play a crucial role in the development of hyperproliferative diseases in the mammary gland.
Copyright 2010 AACR.
Figures



References
-
- Yanez-Mo M, Barreiro O, Gordon-Alonso M, et al. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–46. - PubMed
-
- Charrin S, Le NF, Silvie O, et al. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 2009;420:133–54. - PubMed
-
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. - PubMed
-
- Berditchevski F, Odintsova E. Tetraspanins as regulators of protein trafficking. Traffic. 2007;8:89–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

