Uric acid promotes an acute inflammatory response to sterile cell death in mice
- PMID: 20501947
- PMCID: PMC2877935
- DOI: 10.1172/JCI40124
Uric acid promotes an acute inflammatory response to sterile cell death in mice
Abstract
Necrosis stimulates inflammation, and this response is medically relevant because it contributes to the pathogenesis of a number of diseases. It is thought that necrosis stimulates inflammation because dying cells release proinflammatory molecules that are recognized by the immune system. However, relatively little is known about the molecular identity of these molecules and their contribution to responses in vivo. Here, we investigated the role of uric acid in the inflammatory response to necrotic cells in mice. We found that dead cells not only released intracellular stores of uric acid but also produced it in large amounts postmortem as nucleic acids were degraded. Using newly developed Tg mice that have reduced levels of uric acid either intracellularly and/or extracellularly, we found that uric acid depletion substantially reduces the cell death-induced inflammatory response. Similar results were obtained with pharmacological treatments that reduced uric acid levels either by blocking its synthesis or hydrolyzing it in the extracellular fluids. Importantly, uric acid depletion selectively inhibited the inflammatory response to dying cells but not to microbial molecules or sterile irritant particles. Collectively, our data identify uric acid as a proinflammatory molecule released from dying cells that contributes significantly to the cell death-induced inflammatory responses in vivo.
Figures
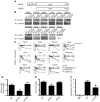
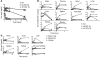
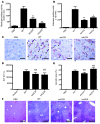
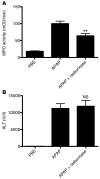
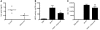
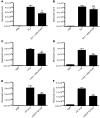
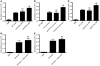
Comment in
- J Clin Invest. 120:1809.
References
-
- Acute myocardial infarction. In: Braunwald E, Zipes DP, and Libby P, eds.Heart Disease: A Textbook of Cardiovascular Medicine . 6th ed. Philadelphia, PA: WB Saunders; 2001:1114–1231.
-
- Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67(5):1016–1023. - PubMed
-
- Sawa Y, et al. Leukocyte depletion attenuates reperfusion injury in patients with left ventricular hypertrophy. Circulation. 1996;93(9):1640–1646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

