The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice
- PMID: 20501949
- PMCID: PMC2877926
- DOI: 10.1172/JCI38369
The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice
Abstract
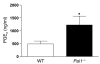
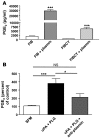
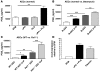
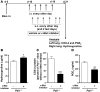
Plasminogen activation to plasmin protects from lung fibrosis, but the mechanism underlying this antifibrotic effect remains unclear. We found that mice lacking plasminogen activation inhibitor-1 (PAI-1), which are protected from bleomycin-induced pulmonary fibrosis, exhibit lung overproduction of the antifibrotic lipid mediator prostaglandin E2 (PGE2). Plasminogen activation upregulated PGE2 synthesis in alveolar epithelial cells, lung fibroblasts, and lung fibrocytes from saline- and bleomycin-treated mice, as well as in normal fetal and adult primary human lung fibroblasts. This response was exaggerated in cells from Pai1-/- mice. Although enhanced PGE2 formation required the generation of plasmin, it was independent of proteinase-activated receptor 1 (PAR-1) and instead reflected proteolytic activation and release of HGF with subsequent induction of COX-2. That the HGF/COX-2/PGE2 axis mediates in vivo protection from fibrosis in Pai1-/- mice was demonstrated by experiments showing that a selective inhibitor of the HGF receptor c-Met increased lung collagen to WT levels while reducing COX-2 protein and PGE2 levels. Of clinical interest, fibroblasts from patients with idiopathic pulmonary fibrosis were found to be defective in their ability to induce COX-2 and, therefore, unable to upregulate PGE2 synthesis in response to plasmin or HGF. These studies demonstrate crosstalk between plasminogen activation and PGE2 generation in the lung and provide a mechanism for the well-known antifibrotic actions of the fibrinolytic pathway.
Figures
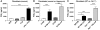
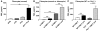

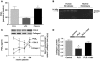



References
-
- Bertozzi P, et al. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990;322(13):890–897. - PubMed
-
- Chapman HA, Allen CL, Stone OL. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis. 1986;133(3):437–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL094311/HL/NHLBI NIH HHS/United States
- R01 AI065543/AI/NIAID NIH HHS/United States
- R56 AI065543/AI/NIAID NIH HHS/United States
- HL058897/HL/NHLBI NIH HHS/United States
- HL078871/HL/NHLBI NIH HHS/United States
- R01 HL087846/HL/NHLBI NIH HHS/United States
- HL094657/HL/NHLBI NIH HHS/United States
- HL087846/HL/NHLBI NIH HHS/United States
- AI065543/AI/NIAID NIH HHS/United States
- HL094311/HL/NHLBI NIH HHS/United States
- K08 HL094657/HL/NHLBI NIH HHS/United States
- R01 HL058897/HL/NHLBI NIH HHS/United States
- R56 HL058897/HL/NHLBI NIH HHS/United States
- R01 HL078871/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

