Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome
- PMID: 20502449
- PMCID: PMC3786710
- DOI: 10.1038/ajg.2010.200
Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome
Abstract
Objectives: The Rome III classification system treats functional constipation (FC) and irritable bowel syndrome with constipation (IBS-C) as distinct disorders, but this distinction appears artificial, and the same drugs are used to treat both. This study's hypothesis is that FC and IBS-C defined by Rome III are not distinct entities.
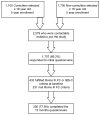
Methods: In all, 1,100 adults with a primary care visit for constipation and 1,700 age- and gender-matched controls from a health maintenance organization completed surveys 12 months apart; 66.2% returned the first questionnaire. Rome III criteria identified 231 with FC and 201 with IBS-C. The second survey was completed by 195 of the FC and 141 of the IBS-C cohorts. Both surveys assessed the severity of constipation and IBS, quality of life (QOL), and psychological distress.
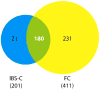
Results: (i) Overlap: if the Rome III requirement that patients meeting criteria for IBS cannot be diagnosed with FC is suspended, 89.5% of IBS-C cases meet criteria for FC and 43.8% of FC patients fulfill criteria for IBS-C. (ii) No qualitative differences between FC and IBS-C: 44.8% of FC patients report abdominal pain, and paradoxically IBS-C patients have more constipation symptoms than FC. (iii) Switching between diagnoses: by 12 months, 1/3 of FC transition to IBS-C and 1/3 of IBS-C change to FC.
Conclusions: Patients identified by Rome III criteria for FC and IBS-C are not distinct groups. Revisions to the Rome III criteria, possibly including incorporation of physiological tests of transit and pelvic floor function, are needed.
Conflict of interest statement
William E. Whitehead has served on the board of the Rome Foundation since 1990. He has also served on advisory boards for Novartis Pharmaceuticals, Takeda Pharmaceuticals, and Ironwoods, each of which has developed and/or marketed drugs for the treatment of functional constipation and irritable bowel syndrome with constipation. He has been the recipient of research grants from Novartis and Takeda pharmaceutical companies. None of the other authors have competing interests.
Figures
Comment in
-
Overlap among the functional gastrointestinal disorders.Am J Gastroenterol. 2010 Nov;105(11):2512. doi: 10.1038/ajg.2010.353. Am J Gastroenterol. 2010. PMID: 21048695 No abstract available.
References
-
- Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE. Rome III: The Functional Gastrointestinal Disorders. McLean, Virginia: Degnon Associates; 2006.
-
- Locke GR, III, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterol. 2000;119:1766–1778. - PubMed
-
- Thompson WG . the Working Team for Functional Bowel Disorders. Functional bowel disorders and functional abdominal pain. In: Drossman DA, Richter JE, Talley NJ, Thompson WG, Corazziari E, Whitehead WE, editors. The functional gastrointestinal disorders. Boston: Little, Brown and Company; 1994. pp. 115–173.
-
- Kamm MA, Muller-Lissner S, Talley NJ, Tack J, Boeckxstaens G, Minushkin ON, Kalinin A, Dzieniszewski J, Haeck P, Fordham F, Hugot-Cournez S, Nault B. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362–372. - PubMed
-
- Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–2354. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical