Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age
- PMID: 20502519
- PMCID: PMC2872642
- DOI: 10.1371/journal.pbio.1000372
Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age
Abstract
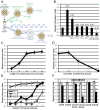
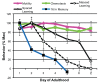
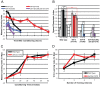
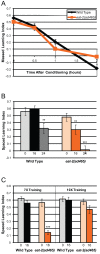
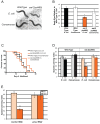
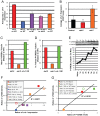
Of all the age-related declines, memory loss is one of the most devastating. While conditions that increase longevity have been identified, the effects of these longevity-promoting factors on learning and memory are unknown. Here we show that the C. elegans Insulin/IGF-1 receptor mutant daf-2 improves memory performance early in adulthood and maintains learning ability better with age but, surprisingly, demonstrates no extension in long-term memory with age. By contrast, eat-2 mutants, a model of Dietary Restriction (DR), exhibit impaired long-term memory in young adulthood but maintain this level of memory longer with age. We find that crh-1, the C. elegans homolog of the CREB transcription factor, is required for long-term associative memory, but not for learning or short-term memory. The expression of crh-1 declines with age and differs in the longevity mutants, and CREB expression and activity correlate with memory performance. Our results suggest that specific longevity treatments have acute and long-term effects on cognitive functions that decline with age through their regulation of rate-limiting genes required for learning and memory.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Learning and memory: ageing without forgetting.Nat Rev Neurosci. 2010 Jul;11(7):456-7. doi: 10.1038/nrn2869. Epub 2010 Jun 9. Nat Rev Neurosci. 2010. PMID: 21467991 No abstract available.
References
-
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. - PubMed
-
- Wood J. G, Rogina B, Lavu S, Howitz K, Helfand S. L, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. - PubMed
-
- Bluher M, Kahn B. B, Kahn C. R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

