IL-4Ralpha-independent expression of mannose receptor and Ym1 by macrophages depends on their IL-10 responsiveness
- PMID: 20502521
- PMCID: PMC2872644
- DOI: 10.1371/journal.pntd.0000689
IL-4Ralpha-independent expression of mannose receptor and Ym1 by macrophages depends on their IL-10 responsiveness
Abstract
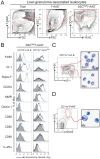
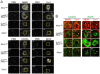
IL-4Ralpha-dependent responses are essential for granuloma formation and host survival during acute schistosomiasis. Previously, we demonstrated that mice deficient for macrophage-specific IL-4Ralpha (LysM(cre)Il4ra(-/lox)) developed increased hepatotoxicity and gut inflammation; whereas inflammation was restricted to the liver of mice lacking T cell-specific IL-4Ralpha expression (iLck(cre)Il4ra(-/lox)). In the study presented here we further investigated their role in liver granulomatous inflammation. Frequencies and numbers of macrophage, lymphocyte or granulocyte populations, as well as Th1/Th2 cytokine responses were similar in Schistosoma mansoni-infected LysM(cre)Il4ra(-/lox) liver granulomas, when compared to Il4ra(-/lox) control mice. In contrast, a shift to Th1 responses with high IFN-gamma and low IL-4, IL-10 and IL-13 was observed in the severely disrupted granulomas of iLck(cre)Il4ra(-/lox) and Il4ra(-/-) mice. As expected, alternative macrophage activation was reduced in both LysM(cre)Il4ra(-/lox) and iLck(cre)Il4ra(-/lox) granulomas with low arginase 1 and heightened nitric oxide synthase RNA expression in granuloma macrophages of both mouse strains. Interestingly, a discrete subpopulation of SSC(high)CD11b+I-A/I-E(high)CD204+ macrophages retained expression of mannose receptor (MMR) and Ym1 in LysM(cre)Il4ra(-/lox) but not in iLck(cre)Il4ra(-/lox) granulomas. While aaMphi were in close proximity to the parasite eggs in Il4ra(-/lox) control mice, MMR+Ym1+ macrophages in LysM(cre)Il4ra(-/lox) mice were restricted to the periphery of the granuloma, indicating that they might have different functions. In vivo IL-10 neutralisation resulted in the disappearance of MMR+Ym1+ macrophages in LysM(cre)Il4ra(-/lox) mice. Together, these results show that IL-4Ralpha-responsive T cells are essential to drive alternative macrophage activation and to control granulomatous inflammation in the liver. The data further suggest that in the absence of macrophage-specific IL-4Ralpha signalling, IL-10 is able to drive mannose receptor- and Ym1-positive macrophages, associated with control of hepatic granulomatous inflammation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
IL-4Ralpha responsiveness of non-CD4 T cells contributes to resistance in schistosoma mansoni infection in pan-T cell-specific IL-4Ralpha-deficient mice.Am J Pathol. 2009 Aug;175(2):706-16. doi: 10.2353/ajpath.2009.090137. Epub 2009 Jul 23. Am J Pathol. 2009. PMID: 19628763 Free PMC article.
-
Control of Schistosoma mansoni egg-induced inflammation by IL-4-responsive CD4(+)CD25(-)CD103(+)Foxp3(-) cells is IL-10-dependent.Eur J Immunol. 2010 Oct;40(10):2837-47. doi: 10.1002/eji.200940075. Eur J Immunol. 2010. PMID: 20821727
-
Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing T(h)2 response.Int Immunol. 2013 Aug;25(8):459-70. doi: 10.1093/intimm/dxt003. Epub 2013 Mar 26. Int Immunol. 2013. PMID: 23532373
-
Current concepts in granulomatous immune responses.Biol Futur. 2021 Mar;72(1):61-68. doi: 10.1007/s42977-021-00077-1. Epub 2021 Feb 25. Biol Futur. 2021. PMID: 34095894 Free PMC article. Review.
-
Macrophage activation governs schistosomiasis-induced inflammation and fibrosis.Eur J Immunol. 2011 Sep;41(9):2509-14. doi: 10.1002/eji.201141869. Eur J Immunol. 2011. PMID: 21952807 Free PMC article. Review.
Cited by
-
Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease.Annu Rev Immunol. 2013;31:317-43. doi: 10.1146/annurev-immunol-032712-095906. Epub 2013 Jan 3. Annu Rev Immunol. 2013. PMID: 23298208 Free PMC article. Review.
-
Monocyte and Macrophage-Mediated Pathology and Protective Immunity During Schistosomiasis.Front Microbiol. 2020 Aug 12;11:1973. doi: 10.3389/fmicb.2020.01973. eCollection 2020. Front Microbiol. 2020. PMID: 32922381 Free PMC article. Review.
-
Th2 responses in schistosomiasis.Semin Immunopathol. 2012 Nov;34(6):863-71. doi: 10.1007/s00281-012-0354-4. Epub 2012 Nov 9. Semin Immunopathol. 2012. PMID: 23139101 Review.
-
Suppressor of Cytokine Signaling 3 in Macrophages Prevents Exacerbated Interleukin-6-Dependent Arginase-1 Activity and Early Permissiveness to Experimental Tuberculosis.Front Immunol. 2017 Nov 10;8:1537. doi: 10.3389/fimmu.2017.01537. eCollection 2017. Front Immunol. 2017. PMID: 29176982 Free PMC article.
-
IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1.J Exp Med. 2013 Oct 21;210(11):2477-91. doi: 10.1084/jem.20121999. Epub 2013 Oct 7. J Exp Med. 2013. PMID: 24101381 Free PMC article.
References
-
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. - PubMed
-
- King CH. Toward the elimination of schistosomiasis. N Engl J Med. 2009;360:106–109. - PubMed
-
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. - PubMed
-
- Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. - PubMed
-
- Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

