Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera
- PMID: 20502538
- PMCID: PMC2836210
- DOI: 10.3114/sim.2010.65.01
Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera
Abstract
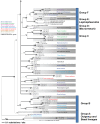
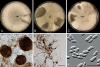
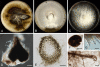
Fungal taxonomists routinely encounter problems when dealing with asexual fungal species due to poly- and paraphyletic generic phylogenies, and unclear species boundaries. These problems are aptly illustrated in the genus Phoma. This phytopathologically significant fungal genus is currently subdivided into nine sections which are mainly based on a single or just a few morphological characters. However, this subdivision is ambiguous as several of the section-specific characters can occur within a single species. In addition, many teleomorph genera have been linked to Phoma, three of which are recognised here. In this study it is attempted to delineate generic boundaries, and to come to a generic circumscription which is more correct from an evolutionary point of view by means of multilocus sequence typing. Therefore, multiple analyses were conducted utilising sequences obtained from 28S nrDNA (Large Subunit - LSU), 18S nrDNA (Small Subunit - SSU), the Internal Transcribed Spacer regions 1 & 2 and 5.8S nrDNA (ITS), and part of the beta-tubulin (TUB) gene region. A total of 324 strains were included in the analyses of which most belonged to Phoma taxa, whilst 54 to related pleosporalean fungi. In total, 206 taxa were investigated, of which 159 are known to have affinities to Phoma. The phylogenetic analysis revealed that the current Boeremaean subdivision is incorrect from an evolutionary point of view, revealing the genus to be highly polyphyletic. Phoma species are retrieved in six distinct clades within the Pleosporales, and appear to reside in different families. The majority of the species, however, including the generic type, clustered in a recently established family, Didymellaceae. In the second part of this study, the phylogenetic variation of the species and varieties in this clade was further assessed. Next to the genus Didymella, which is considered to be the sole teleomorph of Phoma s. str., we also retrieved taxa belonging to the teleomorph genera Leptosphaerulina and Macroventuria in this clade. Based on the sequence data obtained, the Didymellaceae segregate into at least 18 distinct clusters, of which many can be associated with several specific taxonomic characters. Four of these clusters were defined well enough by means of phylogeny and morphology, so that the associated taxa could be transferred to separate genera. Aditionally, this study addresses the taxonomic description of eight species and two varieties that are novel to science, and the recombination of 61 additional taxa.
Keywords: Boeremia; DNA phylogeny; Didymella; Didymellaceae; Epicoccum; Leptosphaerulina; Macroventuria; Peyronellaea; Phoma; Pleosporales; Stagonosporopsis; coelomycetes; taxonomy.
Figures















References
-
- Aa HA van der (1971). Macroventuria, a new genus in the Venturiaceae. Persoonia 6: 359–363.
-
- Aa HA van der (1973). Studies in Phyllosticta I. Studies in Mycology 5: 1–110.
-
- Aa HA van der, Kesteren HA van (1979). Some pycnidial fungi occurring on Atriplex and Chenopodium. Persoonia 10: 267–276.
-
- Aa HA van der, Kesteren HA van (1980). Phoma heteromorphospora nom. nov. Persoonia 10: 267–276.
-
- Aa HA van der, Vanev S (2002). A revision of the species described in Phyllosticta. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
