Synthesis and Evaluation of Chitosan-Vitamin C complex
- PMID: 20502541
- PMCID: PMC2865807
- DOI: 10.4103/0250-474X.57284
Synthesis and Evaluation of Chitosan-Vitamin C complex
Abstract
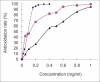
Chitosan is a biocompatible, biodegradable and non-toxic polysaccharide polymer. It dissolves in water only if the pH is lower than 6.5. To extend its range of application, many water-soluble derivatives have therefore been prepared. In this research, chitosan-vitamin C complex was synthesized and characterized with Fourier transformed infrared spectroscopy, differential scanning calorimetry and (1)H-NMR. The solubility of chitosan-vitamin C complex in distilled water was greatly improved. The *O(2) (-) scavenging activity of chitosan-vitamin C complex was compared with chitosan and vitamin C by measuring the auto-oxidation rate of pyrogallic acid. Results showed that the scavenging activity on *O(2) (-) by chitosan-vitamin C complex was stronger than that by CS. At low concentrations (< 0.05 mg/ml), the scavenging activity of chitosan-vitamin C complex was stronger than that of vitamin C, but after certain concentrations (>0.1mg/ml), its scavenging activity was lower than that of vitamin C.
Keywords: Polymer; antioxidation; chitosan; intrinsic viscosity; vitamin C.
Figures





Similar articles
-
Preparation, characterization and antibacterial activity of new ionized chitosan.Carbohydr Polym. 2022 Aug 15;290:119490. doi: 10.1016/j.carbpol.2022.119490. Epub 2022 Apr 18. Carbohydr Polym. 2022. PMID: 35550774
-
Chemical modification of chitosan under high-intensity ultrasound.Ultrason Sonochem. 2005 Jan;12(1-2):95-8. doi: 10.1016/j.ultsonch.2004.03.005. Ultrason Sonochem. 2005. PMID: 15474959
-
A new synthetic methodology for the preparation of biocompatible and organo-soluble barbituric- and thiobarbituric acid based chitosan derivatives for biomedical applications.Mater Sci Eng C Mater Biol Appl. 2016 Sep 1;66:156-163. doi: 10.1016/j.msec.2016.04.056. Epub 2016 Apr 20. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27207049
-
Synthesis and characterization of water soluble O-carboxymethyl chitosan Schiff bases and Cu(II) complexes.Int J Biol Macromol. 2015 Jan;72:94-103. doi: 10.1016/j.ijbiomac.2014.07.029. Epub 2014 Aug 13. Int J Biol Macromol. 2015. PMID: 25128824
-
Trimethyl chitosan and its applications in drug delivery.J Mater Sci Mater Med. 2009 May;20(5):1057-79. doi: 10.1007/s10856-008-3659-z. Epub 2008 Dec 27. J Mater Sci Mater Med. 2009. PMID: 19112609 Review.
Cited by
-
Synthesis and Optimization of Chitosan Nanoparticles Loaded with L-Ascorbic Acid and Thymoquinone.Nanomaterials (Basel). 2018 Nov 7;8(11):920. doi: 10.3390/nano8110920. Nanomaterials (Basel). 2018. PMID: 30405074 Free PMC article.
-
Influence of the Location of Ascorbic Acid in Walnut Oil Spray-Dried Microparticles with Outer Layer on the Physical Characteristics and Oxidative Stability.Antioxidants (Basel). 2020 Dec 14;9(12):1272. doi: 10.3390/antiox9121272. Antioxidants (Basel). 2020. PMID: 33327590 Free PMC article.
-
Development and Optimization of Chitosan-Ascorbate-Based Mucoadhesive Films for Buccal Delivery of Captopril.Pharmaceutics. 2025 Mar 22;17(4):401. doi: 10.3390/pharmaceutics17040401. Pharmaceutics. 2025. PMID: 40284399 Free PMC article.
-
Ferrous ascorbate non-effervescent floating mini-caplets as an oral iron supplement.Drug Deliv Transl Res. 2025 May;15(5):1554-1566. doi: 10.1007/s13346-024-01691-x. Epub 2024 Aug 12. Drug Deliv Transl Res. 2025. PMID: 39133426
-
Encapsulation and controlled release of vitamin C in modified cellulose nanocrystal/chitosan nanocapsules.Curr Res Food Sci. 2021 Apr 2;4:215-223. doi: 10.1016/j.crfs.2021.03.010. eCollection 2021. Curr Res Food Sci. 2021. PMID: 33937869 Free PMC article.
References
-
- Ravi Kumar MN. A review of chitin and CS applications. React Funct Polym. 2000;46:1–27.
-
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on CS-based micro- and nanoparticles in drug delivery. J Controlled Release. 2004;100:5–28. - PubMed
-
- Dodane V, Vilivalam VD. Pharmaceutical applications of CS. Pharm Sci Technol Today. 1998;1:246–53.
-
- Dyer AM, Hinchcliffe M, Watts P. Nasal delivery of insulin using novel CS based formulations: A comparative study in two animal models between simple CS formulations and CS nanoparticles. Pharm Res. 2002;19:998–1008. - PubMed
-
- Hoven VP, Tangpasuthadol V, Angkitpaiboon Y, Vallapab N, Kiatkamjornwongc S. Surface-charged CS: Preparation and protein adsorption. Carbohydr Polym. 2007;68:44–53.
LinkOut - more resources
Full Text Sources
Other Literature Sources
