Leishmania donovani isolates with antimony-resistant but not -sensitive phenotype inhibit sodium antimony gluconate-induced dendritic cell activation
- PMID: 20502630
- PMCID: PMC2873921
- DOI: 10.1371/journal.ppat.1000907
Leishmania donovani isolates with antimony-resistant but not -sensitive phenotype inhibit sodium antimony gluconate-induced dendritic cell activation
Abstract
The inability of sodium antimony gluconate (SAG)-unresponsive kala-azar patients to clear Leishmania donovani (LD) infection despite SAG therapy is partly due to an ill-defined immune-dysfunction. Since dendritic cells (DCs) typically initiate anti-leishmanial immunity, a role for DCs in aberrant LD clearance was investigated. Accordingly, regulation of SAG-induced activation of murine DCs following infection with LD isolates exhibiting two distinct phenotypes such as antimony-resistant (Sb(R)LD) and antimony-sensitive (Sb(S)LD) was compared in vitro. Unlike Sb(S)LD, infection of DCs with Sb(R)LD induced more IL-10 production and inhibited SAG-induced secretion of proinflammatory cytokines, up-regulation of co-stimulatory molecules and leishmanicidal effects. Sb(R)LD inhibited these effects of SAG by blocking activation of PI3K/AKT and NF-kappaB pathways. In contrast, Sb(S)LD failed to block activation of SAG (20 microg/ml)-induced PI3K/AKT pathway; which continued to stimulate NF-kappaB signaling, induce leishmanicidal effects and promote DC activation. Notably, prolonged incubation of DCs with Sb(S)LD also inhibited SAG (20 microg/ml)-induced activation of PI3K/AKT and NF-kappaB pathways and leishmanicidal effects, which was restored by increasing the dose of SAG to 40 microg/ml. In contrast, Sb(R)LD inhibited these SAG-induced events regardless of duration of DC exposure to Sb(R)LD or dose of SAG. Interestingly, the inhibitory effects of isogenic Sb(S)LD expressing ATP-binding cassette (ABC) transporter MRPA on SAG-induced leishmanicidal effects mimicked that of Sb(R)LD to some extent, although antimony resistance in clinical LD isolates is known to be multifactorial. Furthermore, NF-kappaB was found to transcriptionally regulate expression of murine gammaglutamylcysteine synthetase heavy-chain (mgammaGCS(hc)) gene, presumably an important regulator of antimony resistance. Importantly, Sb(R)LD but not Sb(S)LD blocked SAG-induced mgammaGCS expression in DCs by preventing NF-kappaB binding to the mgammaGCS(hc) promoter. Our findings demonstrate that Sb(R)LD but not Sb(S)LD prevents SAG-induced DC activation by suppressing a PI3K-dependent NF-kappaB pathway and provide the evidence for differential host-pathogen interaction mediated by Sb(R)LD and Sb(S)LD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
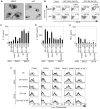






References
-
- Lira R, Sundar S, Makharia A, Kenney R, Gam A, et al. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–567. - PubMed
-
- Ashutosh, Sundar S, Goyal N. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol. 2007;56:143–153. - PubMed
-
- Mukherjee A, Padmanabhan PK, Singh S, Roy G, Girard I, et al. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J Antimicrob Chemother. 2007;59:204–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

