Alzheimer's disease amyloid-beta links lens and brain pathology in Down syndrome
- PMID: 20502642
- PMCID: PMC2873949
- DOI: 10.1371/journal.pone.0010659
Alzheimer's disease amyloid-beta links lens and brain pathology in Down syndrome
Abstract
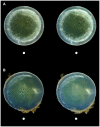
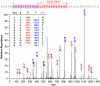
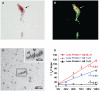
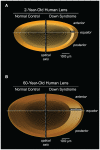
Down syndrome (DS, trisomy 21) is the most common chromosomal disorder and the leading genetic cause of intellectual disability in humans. In DS, triplication of chromosome 21 invariably includes the APP gene (21q21) encoding the Alzheimer's disease (AD) amyloid precursor protein (APP). Triplication of the APP gene accelerates APP expression leading to cerebral accumulation of APP-derived amyloid-beta peptides (Abeta), early-onset AD neuropathology, and age-dependent cognitive sequelae. The DS phenotype complex also includes distinctive early-onset cerulean cataracts of unknown etiology. Previously, we reported increased Abeta accumulation, co-localizing amyloid pathology, and disease-linked supranuclear cataracts in the ocular lenses of subjects with AD. Here, we investigate the hypothesis that related AD-linked Abeta pathology underlies the distinctive lens phenotype associated with DS. Ophthalmological examinations of DS subjects were correlated with phenotypic, histochemical, and biochemical analyses of lenses obtained from DS, AD, and normal control subjects. Evaluation of DS lenses revealed a characteristic pattern of supranuclear opacification accompanied by accelerated supranuclear Abeta accumulation, co-localizing amyloid pathology, and fiber cell cytoplasmic Abeta aggregates (approximately 5 to 50 nm) identical to the lens pathology identified in AD. Peptide sequencing, immunoblot analysis, and ELISA confirmed the identity and increased accumulation of Abeta in DS lenses. Incubation of synthetic Abeta with human lens protein promoted protein aggregation, amyloid formation, and light scattering that recapitulated the molecular pathology and clinical features observed in DS lenses. These results establish the genetic etiology of the distinctive lens phenotype in DS and identify the molecular origin and pathogenic mechanism by which lens pathology is expressed in this common chromosomal disorder. Moreover, these findings confirm increased Abeta accumulation as a key pathogenic determinant linking lens and brain pathology in both DS and AD.
Conflict of interest statement
Figures





Similar articles
-
Alzheimer's disease amyloid-β pathology in the lens of the eye.Exp Eye Res. 2022 Aug;221:108974. doi: 10.1016/j.exer.2022.108974. Epub 2022 Feb 21. Exp Eye Res. 2022. PMID: 35202705 Free PMC article.
-
In vivo quasi-elastic light scattering detects molecular changes in the lenses of adolescents with Down syndrome.Exp Eye Res. 2024 Apr;241:109818. doi: 10.1016/j.exer.2024.109818. Epub 2024 Feb 2. Exp Eye Res. 2024. PMID: 38422787
-
Characterization of monomeric and soluble aggregated Aβ in Down's syndrome and Alzheimer's disease brains.Neurosci Lett. 2021 May 29;754:135894. doi: 10.1016/j.neulet.2021.135894. Epub 2021 Apr 10. Neurosci Lett. 2021. PMID: 33848613
-
[Elucidating Pathogenic Mechanisms of Early-onset Alzheimer's Disease in Down Syndrome Patients].Yakugaku Zasshi. 2017;137(7):801-805. doi: 10.1248/yakushi.16-00236-2. Yakugaku Zasshi. 2017. PMID: 28674290 Review. Japanese.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Self-assembly of protein aggregates in ageing disorders: the lens and cataract model.Philos Trans R Soc Lond B Biol Sci. 2013 Mar 25;368(1617):20120104. doi: 10.1098/rstb.2012.0104. Print 2013 May 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23530262 Free PMC article.
-
Alzheimer's disease diagnosis by detecting exogenous fluorescent signal of ligand bound to Beta amyloid in the lens of human eye: an exploratory study.Front Neurol. 2013 May 27;4:62. doi: 10.3389/fneur.2013.00062. eCollection 2013. Front Neurol. 2013. PMID: 23750151 Free PMC article.
-
The Eye As a Biomarker for Alzheimer's Disease.Front Neurosci. 2016 Nov 17;10:536. doi: 10.3389/fnins.2016.00536. eCollection 2016. Front Neurosci. 2016. PMID: 27909396 Free PMC article. Review.
-
Ocular manifestations of Alzheimer's disease in animal models.Int J Alzheimers Dis. 2012;2012:786494. doi: 10.1155/2012/786494. Epub 2012 May 15. Int J Alzheimers Dis. 2012. PMID: 22666623 Free PMC article.
-
Cataract surgery outcomes in pediatric patients with systemic comorbidities.Indian J Ophthalmol. 2023 Jan;71(1):125-137. doi: 10.4103/ijo.IJO_1465_22. Indian J Ophthalmol. 2023. PMID: 36588222 Free PMC article.
References
-
- Down JLH. Observations on an ethnic classification of idiots. London Hosp Clin Lect Rep. 1866;3:259.
-
- Epstein CJ. Down syndrome, trisomy 21. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic Basis of Inherited Disease. New York: McGraw-Hill; 1989. pp. 291–326.
-
- Online Mendelian Inheritance in Man - Down Syndrome. Available: http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=190685. Accessed 25 April 2010.
-
- Pueschel SM. Clinical aspects of Down syndrome from infancy to adulthood. Am J Med Genet. 1990;Suppl 7:52–56. - PubMed
-
- Roizen NJ, Patterson D. Down's syndrome. The Lancet. 2003;361:1281–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

