The C-F bond as a conformational tool in organic and biological chemistry
- PMID: 20502650
- PMCID: PMC2874311
- DOI: 10.3762/bjoc.6.38
The C-F bond as a conformational tool in organic and biological chemistry
Abstract
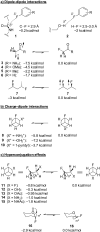
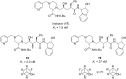
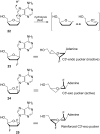
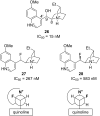
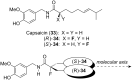
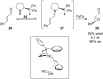
Organofluorine compounds are widely used in many different applications, ranging from pharmaceuticals and agrochemicals to advanced materials and polymers. It has been recognised for many years that fluorine substitution can confer useful molecular properties such as enhanced stability and hydrophobicity. Another impact of fluorine substitution is to influence the conformations of organic molecules. The stereoselective introduction of fluorine atoms can therefore be exploited as a conformational tool for the synthesis of shape-controlled functional molecules. This review will begin by describing some general aspects of the C-F bond and the various conformational effects associated with C-F bonds (i.e. dipole-dipole interactions, charge-dipole interactions and hyperconjugation). Examples of functional molecules that exploit these conformational effects will then be presented, drawing from a diverse range of molecules including pharmaceuticals, organocatalysts, liquid crystals and peptides.
Keywords: conformation; functional molecules; organofluorine chemistry; stereochemistry; stereoelectronic effects.
Figures
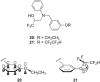

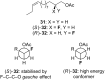



References
-
- Kirsch P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications. Weinheim, Germany: Wiley-VCH; 2004. - DOI
-
- Smith M B, March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 5th ed. New York, USA: Wiley-Interscience; 2001.
LinkOut - more resources
Full Text Sources
