Differentiating embryonic stem cells pass through 'temporal windows' that mark responsiveness to exogenous and paracrine mesendoderm inducing signals
- PMID: 20502661
- PMCID: PMC2873409
- DOI: 10.1371/journal.pone.0010706
Differentiating embryonic stem cells pass through 'temporal windows' that mark responsiveness to exogenous and paracrine mesendoderm inducing signals
Abstract
Background: Mesendoderm induction during embryonic stem cell (ESC) differentiation in vitro is stimulated by the Transforming Growth Factor and Wingless (Wnt) families of growth factors.
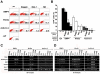
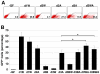
Principal findings: We identified the periods during which Bone Morphogenetic Protein (BMP) 4, Wnt3a or Activin A were able to induce expression of the mesendoderm marker, Mixl1, in differentiating mouse ESCs. BMP4 and Wnt3a were required between differentiation day (d) 1.5 and 3 to most effectively induce Mixl1, whilst Activin A induced Mixl1 expression in ESC when added between d2 and d4, indicating a subtle difference in the requirement for Activin receptor signalling in this process. Stimulation of ESCs with these factors at earlier or later times resulted in little Mixl1 induction, suggesting that the differentiating ESCs passed through 'temporal windows' in which they sequentially gained and lost competence to respond to each growth factor. Inhibition of either Activin or Wnt signalling blocked Mixl1 induction by any of the three mesendoderm-inducing factors. Mixing experiments in which chimeric EBs were formed between growth factor-treated and untreated ESCs revealed that BMP, Activin and Wnt signalling all contributed to the propagation of paracrine mesendoderm inducing signals between adjacent cells. Finally, we demonstrated that the differentiating cells passed through 'exit gates' after which point they were no longer dependent on signalling from inducing molecules for Mixl1 expression.
Conclusions: These studies suggest that differentiating ESCs are directed by an interconnected network of growth factors similar to those present in early embryos and that the timing of growth factor activity is critical for mesendoderm induction.
Conflict of interest statement
Figures




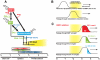
Similar articles
-
Microbioreactor arrays for full factorial screening of exogenous and paracrine factors in human embryonic stem cell differentiation.PLoS One. 2012;7(12):e52405. doi: 10.1371/journal.pone.0052405. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300662 Free PMC article.
-
Combined activin A/LiCl/Noggin treatment improves production of mouse embryonic stem cell-derived definitive endoderm cells.J Cell Biochem. 2011 Apr;112(4):1022-34. doi: 10.1002/jcb.22962. J Cell Biochem. 2011. PMID: 21400570
-
Activation of FGFR(IIIc) isoforms promotes activin-induced mesendoderm development in mouse embryonic stem cells and reduces Sox17 coexpression in EpCAM+ cells.Stem Cell Res. 2011 May;6(3):262-75. doi: 10.1016/j.scr.2011.02.003. Epub 2011 Feb 27. Stem Cell Res. 2011. PMID: 21513905
-
Signaling pathways during maintenance and definitive endoderm differentiation of embryonic stem cells.Int J Dev Biol. 2013;57(1):1-12. doi: 10.1387/ijdb.120115ls. Int J Dev Biol. 2013. PMID: 23585347 Review.
-
Differential response of epiblast stem cells to Nodal and Activin signalling: a paradigm of early endoderm development in the embryo.Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130550. doi: 10.1098/rstb.2013.0550. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349457 Free PMC article. Review.
Cited by
-
Pluripotency factors in embryonic stem cells regulate differentiation into germ layers.Cell. 2011 Jun 10;145(6):875-89. doi: 10.1016/j.cell.2011.05.017. Cell. 2011. PMID: 21663792 Free PMC article.
-
Thalidomide induces apoptosis in undifferentiated human induced pluripotent stem cells.In Vitro Cell Dev Biol Anim. 2017 Oct;53(9):841-851. doi: 10.1007/s11626-017-0192-8. Epub 2017 Aug 28. In Vitro Cell Dev Biol Anim. 2017. PMID: 28849348
-
Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers.J Am Soc Nephrol. 2014 Jun;25(6):1211-25. doi: 10.1681/ASN.2013080831. Epub 2013 Dec 19. J Am Soc Nephrol. 2014. PMID: 24357672 Free PMC article.
-
Generation and trapping of a mesoderm biased state of human pluripotency.Nat Commun. 2020 Oct 5;11(1):4989. doi: 10.1038/s41467-020-18727-8. Nat Commun. 2020. PMID: 33020476 Free PMC article.
-
Microbioreactor arrays for full factorial screening of exogenous and paracrine factors in human embryonic stem cell differentiation.PLoS One. 2012;7(12):e52405. doi: 10.1371/journal.pone.0052405. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300662 Free PMC article.
References
-
- Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. - PubMed
-
- Hirst CE, Ng ES, Azzola L, Voss AK, Thomas T, et al. Transcriptional profiling of mouse and human ES cells identifies SLAIN1, a novel stem cell gene. Dev Biol. 2006;293:90–103. - PubMed
-
- Gadue P, Huber TL, Nostro MC, Kattman S, Keller GM. Germ layer induction from embryonic stem cells. Exp Hematol. 2005;33:955–964. - PubMed
-
- Smith J, Wardle F, Loose M, Stanley E, Patient R. Germ Layer Induction in ESC - Following the Vertebrate Roadmap. Current Protocols in Stem Cell Biology 1 2007 - PubMed
-
- Tam PP, Loebel DA, Tanaka SS. Building the mouse gastrula: signals, asymmetry and lineages. Curr Opin Genet Dev. 2006;16:419–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

