Conserved charged amino acids within Sendai virus C protein play multiple roles in the evasion of innate immune responses
- PMID: 20502666
- PMCID: PMC2873429
- DOI: 10.1371/journal.pone.0010719
Conserved charged amino acids within Sendai virus C protein play multiple roles in the evasion of innate immune responses
Abstract
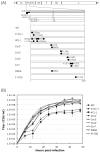
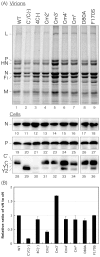
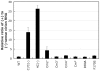
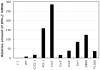
One of the accessory proteins of Sendai virus (SeV), C, translated from an alternate reading frame of P/V mRNA has been shown to function at multiple stages of infection in cell cultures as well as in mice. C protein has been reported to counteract signal transduction by interferon (IFN), inhibit apoptosis induced by the infection, enhance the efficiency of budding of viral particles, and regulate the polarity of viral genome-length RNA synthesis to maximize production of infectious particles. In this study, we have generated a series of SeV recombinants containing substitutions of highly conserved, charged residues within the C protein, and characterized them together with previously-reported C'/C(-), 4C(-), and F170S recombinant viruses in infected cell cultures in terms of viral replication, cytopathogenicity, and antagonizing effects on host innate immunity. Unexpectedly, the amino acid substitutions had no or minimal effect on viral growth and viral RNA synthesis. However, all the substitutions of charged amino acids resulted in the loss of a counteracting effect against the establishment of an IFN-alpha-mediated anti-viral state. Infection by the virus (Cm2') containing mutations at K77 and D80 induced significant IFN-beta production, severe cytopathic effects, and detectable amounts of viral dsRNA production. In addition to the Cm2' virus, the virus containing mutations at E114 and E115 did not inhibit the poly(I:C)-triggered translocation of cellular IRF-3 to the nucleus. These results suggest that the C protein play important roles in viral escape from induction of IFN-beta and cell death triggered by infection by means of counteracting the pathway leading to activation of IRF-3 as well as of minimizing viral dsRNA production.
Conflict of interest statement
Figures




Similar articles
-
A Single Amino Acid Substitution within the Paramyxovirus Sendai Virus Nucleoprotein Is a Critical Determinant for Production of Interferon-Beta-Inducing Copyback-Type Defective Interfering Genomes.J Virol. 2018 Feb 12;92(5):e02094-17. doi: 10.1128/JVI.02094-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237838 Free PMC article.
-
Paramyxovirus Sendai virus C proteins are essential for maintenance of negative-sense RNA genome in virus particles.Virology. 2008 May 10;374(2):495-505. doi: 10.1016/j.virol.2008.01.004. Epub 2008 Feb 5. Virology. 2008. PMID: 18252261
-
Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling.Virology. 2002 Nov 10;303(1):15-32. doi: 10.1006/viro.2002.1738. Virology. 2002. PMID: 12482655
-
[Sendai virus proteins counteracting the host innate immunity].Uirusu. 2004 Dec;54(2):179-88. doi: 10.2222/jsv.54.179. Uirusu. 2004. PMID: 15745155 Review. Japanese.
-
C Proteins: Controllers of Orderly Paramyxovirus Replication and of the Innate Immune Response.Viruses. 2022 Jan 12;14(1):137. doi: 10.3390/v14010137. Viruses. 2022. PMID: 35062341 Free PMC article. Review.
Cited by
-
Structural Insight into the Interaction of Sendai Virus C Protein with Alix To Stimulate Viral Budding.J Virol. 2021 Sep 9;95(19):e0081521. doi: 10.1128/JVI.00815-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34287046 Free PMC article.
-
Oncolytic Rodent Protoparvoviruses Evade a TLR- and RLR-Independent Antiviral Response in Transformed Cells.Pathogens. 2023 Apr 17;12(4):607. doi: 10.3390/pathogens12040607. Pathogens. 2023. PMID: 37111493 Free PMC article.
-
Structural Basis of the Inhibition of STAT1 Activity by Sendai Virus C Protein.J Virol. 2015 Nov;89(22):11487-99. doi: 10.1128/JVI.01887-15. Epub 2015 Sep 2. J Virol. 2015. PMID: 26339056 Free PMC article.
-
A Single Amino Acid Substitution within the Paramyxovirus Sendai Virus Nucleoprotein Is a Critical Determinant for Production of Interferon-Beta-Inducing Copyback-Type Defective Interfering Genomes.J Virol. 2018 Feb 12;92(5):e02094-17. doi: 10.1128/JVI.02094-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237838 Free PMC article.
-
The C proteins of human parainfluenza virus type 1 limit double-stranded RNA accumulation that would otherwise trigger activation of MDA5 and protein kinase R.J Virol. 2011 Feb;85(4):1495-506. doi: 10.1128/JVI.01297-10. Epub 2010 Dec 1. J Virol. 2011. PMID: 21123378 Free PMC article.
References
-
- Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology, 5th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2006. pp. 1449–1496.
-
- Garcin D, Marq JB, Strahle L, le Mercier P, Kolakofsky D. All four Sendai Virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology. 2002;295:256–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

