Seasonal influenza vaccine and protection against pandemic (H1N1) 2009-associated illness among US military personnel
- PMID: 20502705
- PMCID: PMC2873284
- DOI: 10.1371/journal.pone.0010722
Seasonal influenza vaccine and protection against pandemic (H1N1) 2009-associated illness among US military personnel
Abstract
Introduction: A novel A/H1N1 virus is the cause of the present influenza pandemic; vaccination is a key countermeasure, however, few data assessing prior seasonal vaccine effectiveness (VE) against the pandemic strain of H1N1 (pH1N1) virus are available.
Materials and methods: Surveillance of influenza-related medical encounter data of active duty military service members stationed in the United States during the period of April-October 2009 with comparison of pH1N1-confirmed cases and location and date-matched controls. Crude odds ratios (OR) and VE estimates for immunized versus non-immunized were calculated as well as adjusted OR (AOR) controlling for sex, age group, and history of prior influenza vaccination. Separate stratified VE analyses by vaccine type (trivalent inactivated [TIV] or live attenuated [LAIV]), age groups and hospitalization status were also performed. For the period of April 20 to October 15, 2009, a total of 1,205 cases of pH1N1-confirmed cases were reported, 966 (80%) among males and over one-half (58%) under 25 years of age. Overall VE for service members was found to be 45% (95% CI, 33 to 55%). Immunization with prior season's TIV (VE = 44%, 95% CI, 32 to 54%) as well as LAIV (VE = 24%, 95% CI, 6 to 38%) were both found to be associated with protection. Of significance, VE against a severe disease outcome was higher (VE = 62%, 95% CI, 14 to 84%) than against milder outcomes (VE = 42%, 95% CI, 29 to 53%).
Conclusion: A moderate association with protection against clinically apparent, laboratory-confirmed Pandemic (H1N1) 2009-associated illness was found for immunization with either TIV or LAIV 2008-09 seasonal influenza vaccines. This association with protection was found to be especially apparent for severe disease as compared to milder outcome, as well as in the youngest and older populations. Prior vaccination with seasonal influenza vaccines in 2004-08 was also independently associated with protection.
Conflict of interest statement
Figures
Similar articles
-
Effectiveness of seasonal influenza vaccines against influenza-associated illnesses among US military personnel in 2010-11: a case-control approach.PLoS One. 2012;7(7):e41435. doi: 10.1371/journal.pone.0041435. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22859985 Free PMC article.
-
Influenza vaccine effectiveness against influenza-related hospitalization during a season with mixed outbreaks of four influenza viruses: a test-negative case-control study in adults in Canada.BMC Infect Dis. 2017 Dec 29;17(1):805. doi: 10.1186/s12879-017-2905-8. BMC Infect Dis. 2017. PMID: 29284435 Free PMC article.
-
Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel.JAMA. 2009 Mar 4;301(9):945-53. doi: 10.1001/jama.2009.265. Epub 2009 Mar 2. JAMA. 2009. PMID: 19255113
-
The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis.BMC Med. 2019 Jan 10;17(1):9. doi: 10.1186/s12916-018-1239-8. BMC Med. 2019. PMID: 30626399 Free PMC article.
-
Influenza vaccines: from surveillance through production to protection.Mayo Clin Proc. 2010 Mar;85(3):257-73. doi: 10.4065/mcp.2009.0615. Epub 2010 Jan 29. Mayo Clin Proc. 2010. PMID: 20118381 Free PMC article. Review.
Cited by
-
No association between 2008-09 influenza vaccine and influenza A(H1N1)pdm09 virus infection, Manitoba, Canada, 2009.Emerg Infect Dis. 2012 May;18(5):801-10. doi: 10.3201/eid1805.111596. Emerg Infect Dis. 2012. PMID: 22516189 Free PMC article.
-
Effectiveness of seasonal influenza vaccines against influenza-associated illnesses among US military personnel in 2010-11: a case-control approach.PLoS One. 2012;7(7):e41435. doi: 10.1371/journal.pone.0041435. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22859985 Free PMC article.
-
Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques.J Virol. 2013 May;87(10):5512-22. doi: 10.1128/JVI.03030-12. Epub 2013 Mar 6. J Virol. 2013. PMID: 23468501 Free PMC article.
-
Original Antigenic Sin Response to RNA Viruses and Antiviral Immunity.Immune Netw. 2016 Oct;16(5):261-270. doi: 10.4110/in.2016.16.5.261. Epub 2016 Oct 25. Immune Netw. 2016. PMID: 27799871 Free PMC article. Review.
-
Field effectiveness of pandemic and 2009-2010 seasonal vaccines against 2009-2010 A(H1N1) influenza: estimations from surveillance data in France.PLoS One. 2011 May 10;6(5):e19621. doi: 10.1371/journal.pone.0019621. PLoS One. 2011. PMID: 21573005 Free PMC article.
References
-
- Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3–26. - PubMed
-
- WHO. Ad hoc Policy Advisory Working Group on Influenza A (H1N1) vaccines. Geneva: World Health Organization; 2009.
-
- WHO. Summary report of a High-Level Consultation: new influenza A (H1N1) Geneva: World Health Organization; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

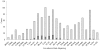
 Light Bars = Non-Hospitalized…
Light Bars = Non-Hospitalized… Dark Bars = Hospitalized Cases.
Dark Bars = Hospitalized Cases.