The effect of high-altitude on human skeletal muscle energetics: P-MRS results from the Caudwell Xtreme Everest expedition
- PMID: 20502713
- PMCID: PMC2873292
- DOI: 10.1371/journal.pone.0010681
The effect of high-altitude on human skeletal muscle energetics: P-MRS results from the Caudwell Xtreme Everest expedition
Abstract
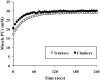
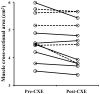
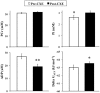
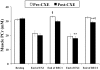
Many disease states are associated with regional or systemic hypoxia. The study of healthy individuals exposed to high-altitude hypoxia offers a way to explore hypoxic adaptation without the confounding effects of disease and therapeutic interventions. Using (31)P magnetic resonance spectroscopy and imaging, we investigated skeletal muscle energetics and morphology after exposure to hypobaric hypoxia in seven altitude-naïve subjects (trekkers) and seven experienced climbers. The trekkers ascended to 5300 m while the climbers ascended above 7950 m. Before the study, climbers had better mitochondrial function (evidenced by shorter phosphocreatine recovery halftime) than trekkers: 16+/-1 vs. 22+/-2 s (mean +/- SE, p<0.01). Climbers had higher resting [Pi] than trekkers before the expedition and resting [Pi] was raised across both groups on their return (PRE: 2.6+/-0.2 vs. POST: 3.0+/-0.2 mM, p<0.05). There was significant muscle atrophy post-CXE (PRE: 4.7+/-0.2 vs. POST: 4.5+/-0.2 cm(2), p<0.05), yet exercising metabolites were unchanged. These results suggest that, in response to high altitude hypoxia, skeletal muscle function is maintained in humans, despite significant atrophy.
Conflict of interest statement
Figures
References
-
- Reynafarje B. Myoglobin content and enzymatic activity of muscle and altitude adaptation. J Appl Physiol. 1962;17:301–305. - PubMed
-
- Hochachka PW, Stanley C, Merkt J, Sumar-Kalinowski J. Metabolic meaning of elevated levels of oxidative enzymes in high altitude adapted animals: an interpretive hypothesis. Respir Physiol. 1983;52:303–313. - PubMed
-
- Lynn EG, Lu Z, Minerbi D, Sack MN. The regulation, control, and consequences of mitochondrial oxygen utilization and disposition in the heart and skeletal muscle during hypoxia. Antioxid Redox Signal. 2007;9:1353–1361. - PubMed
-
- Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. 2001;204:3133–3139. - PubMed
-
- Rosser BW, Hochachka PW. Metabolic capacity of muscle fibers from high-altitude natives. Eur J Appl Physiol Occup Physiol. 1993;67:513–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

