Alkyltransferase-like proteins: molecular switches between DNA repair pathways
- PMID: 20502938
- PMCID: PMC3088651
- DOI: 10.1007/s00018-010-0405-8
Alkyltransferase-like proteins: molecular switches between DNA repair pathways
Abstract
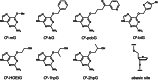
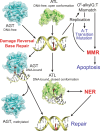
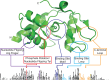
Alkyltransferase-like proteins (ATLs) play a role in the protection of cells from the biological effects of DNA alkylation damage. Although ATLs share functional motifs with the DNA repair protein and cancer chemotherapy target O⁶-alkylguanine-DNA alkyltransferase, they lack the reactive cysteine residue required for alkyltransferase activity, so its mechanism for cell protection was previously unknown. Here we review recent advances in unraveling the enigmatic cellular protection provided by ATLs against the deleterious effects of DNA alkylation damage. We discuss exciting new evidence that ATLs aid in the repair of DNA O⁶-alkylguanine lesions through a novel repair cross-talk between DNA-alkylation base damage responses and the DNA nucleotide excision repair pathway.
Figures

Similar articles
-
Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools.Chem Res Toxicol. 2011 May 16;24(5):618-39. doi: 10.1021/tx200031q. Epub 2011 Apr 28. Chem Res Toxicol. 2011. PMID: 21466232 Free PMC article. Review.
-
Conserved structural motifs governing the stoichiometric repair of alkylated DNA by O(6)-alkylguanine-DNA alkyltransferase.Mutat Res. 2000 Aug 30;460(3-4):151-63. doi: 10.1016/s0921-8777(00)00024-0. Mutat Res. 2000. PMID: 10946226 Review.
-
DNA binding, nucleotide flipping, and the helix-turn-helix motif in base repair by O6-alkylguanine-DNA alkyltransferase and its implications for cancer chemotherapy.DNA Repair (Amst). 2007 Aug 1;6(8):1100-15. doi: 10.1016/j.dnarep.2007.03.011. Epub 2007 May 7. DNA Repair (Amst). 2007. PMID: 17485252 Free PMC article. Review.
-
Flipping of alkylated DNA damage bridges base and nucleotide excision repair.Nature. 2009 Jun 11;459(7248):808-13. doi: 10.1038/nature08076. Nature. 2009. PMID: 19516334 Free PMC article.
-
Insight into the cooperative DNA binding of the O⁶-alkylguanine DNA alkyltransferase.DNA Repair (Amst). 2014 Aug;20:14-22. doi: 10.1016/j.dnarep.2014.01.006. Epub 2014 Feb 16. DNA Repair (Amst). 2014. PMID: 24553127 Free PMC article. Review.
Cited by
-
Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools.Chem Res Toxicol. 2011 May 16;24(5):618-39. doi: 10.1021/tx200031q. Epub 2011 Apr 28. Chem Res Toxicol. 2011. PMID: 21466232 Free PMC article. Review.
-
P53 conformational switching for selectivity may reveal a general solution for specific DNA binding.EMBO J. 2011 Jun 1;30(11):2099-100. doi: 10.1038/emboj.2011.133. EMBO J. 2011. PMID: 21629273 Free PMC article.
-
DNA repair by reversal of DNA damage.Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a012575. doi: 10.1101/cshperspect.a012575. Cold Spring Harb Perspect Biol. 2013. PMID: 23284047 Free PMC article. Review.
-
Alkyltransferase-like protein (eATL) prevents mismatch repair-mediated toxicity induced by O6-alkylguanine adducts in Escherichia coli.Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18050-5. doi: 10.1073/pnas.1008635107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921378 Free PMC article.
-
Alkyltransferase-like protein clusters scan DNA rapidly over long distances and recruit NER to alkyl-DNA lesions.Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9318-9328. doi: 10.1073/pnas.1916860117. Epub 2020 Apr 9. Proc Natl Acad Sci U S A. 2020. PMID: 32273391 Free PMC article.
References
-
- Beranek DT. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res. 1990;231:11–30. - PubMed
-
- Colvin DM. Alkylating agents and platinum antitumor compounds. In: Holland JF, Frei E, Bast RC Jr, Kufe DW, Morton DL, Weichselbaum RR, editors. Cancer medicine. Media: Willams & Wilkins; 1997. pp. 949–975.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

