Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection
- PMID: 20503391
- PMCID: PMC4121077
- DOI: 10.1002/ar.21094
Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection
Abstract
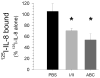
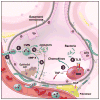
Exposure to viruses and bacteria results in lung infections and places a significant burden on public health. The innate immune system is an early warning system that recognizes viruses and bacteria, which results in the rapid production of inflammatory mediators such as cytokines and chemokines and the pulmonary recruitment of leukocytes. When leukocytes emigrate from the systemic circulation through the extracellular matrix (ECM) in response to lung infection they encounter proteoglycans, which consist of a core protein and their associated glycosaminoglycans. In this review, we discuss how proteoglycans serve to modify the pulmonary inflammatory response and leukocyte migration through a number of different mechanisms including: (1) The ability of soluble proteoglycans or fragments of glycosaminoglycans to activate Toll-like receptor (TLRs) signaling pathways; (2) The binding and sequestration of cytokines, chemokines, and growth factors by proteoglycans; (3) the ability of proteoglycans and hyaluronan to facilitate leukocyte adhesion and sequestration; and (4) The interactions between proteoglycans and matrix metalloproteinases (MMP) that alter the function of these proteases. In conclusion, proteoglycans fine-tune tissue inflammation through a number of different mechanisms. Clarification of the mechanisms whereby proteoglycans modulate the pulmonary inflammatory response will most likely lead to new therapeutic approaches to inflammatory lung disease and lung infection.
Figures





References
-
- Akira S. Innate immunity to pathogens: diversity in receptors for microbial recognition. Immunol Rev. 2009;227:5–8. - PubMed
-
- Ali S, Palmer AC, Banerjee B, Fritchley SJ, Kirby JA. Examination of the Function of RANTES, MIP-1alpha, and MIP-1beta following Interaction with Heparin-like Glycosaminoglycans. J Biol Chem. 2000;275:11721–11727. - PubMed
-
- Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H, Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

