Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina
- PMID: 20503422
- PMCID: PMC3000797
- DOI: 10.1002/cne.22347
Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina
Abstract
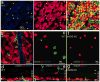
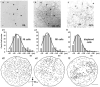
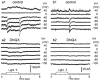
Nitric oxide (NO), produced by NO synthase (NOS), modulates the function of all retinal neurons and ocular blood vessels and participates in the pathogenesis of ocular diseases. To further understand the regulation of ocular NO release, we systematically studied the morphology, topography, and light responses of NOS-containing amacrine cells (NOACs) in dark-adapted mouse retina. Immunohistological staining for neuronal NOS (bNOS), combined with retrograde labeling of ganglion cells (GCs) with Neurobiotin (NB, a gap junction permeable dye) and Lucifer yellow (LY, a less permeable dye), was used to identify NOACs. The light responses of ACs were recorded under whole-cell voltage clamp conditions and cell morphology was examined with a confocal microscope. We found that in dark-adapted conditions bNOS-immunoreactivity (IR) was present primarily in the inner nuclear layer and the ganglion cell layer. bNOS-IR somas were negative for LY, thus they were identified as ACs; nearly 6% of the cells were labeled by NB but not by LY, indicating that they were dye-coupled with GCs. Three morphological subtypes of NOACs (NI, NII, and displaced) were identified. The cell density, intercellular distance, and the distribution of NOACs were studied in whole retinas. Light evoked depolarizing highly sensitive ON-OFF responses in NI cells and less sensitive OFF responses in NII cells. Frequent (1-2 Hz) or abrupt change of light intensity evoked larger peak responses. The possibility for light to modify NO release from NOACs is discussed.
Figures









Similar articles
-
Synaptic circuitry mediating light-evoked signals in dark-adapted mouse retina.Vision Res. 2004 Dec;44(28):3277-88. doi: 10.1016/j.visres.2004.07.045. Vision Res. 2004. PMID: 15535995
-
Morphology and immunoreactivity of retrogradely double-labeled ganglion cells in the mouse retina.Invest Ophthalmol Vis Sci. 2011 Jul 1;52(7):4886-96. doi: 10.1167/iovs.10-5921. Invest Ophthalmol Vis Sci. 2011. PMID: 21482641 Free PMC article.
-
Survey on amacrine cells coupling to retrograde-identified ganglion cells in the mouse retina.Invest Ophthalmol Vis Sci. 2013 Aug 1;54(8):5151-62. doi: 10.1167/iovs.13-11774. Invest Ophthalmol Vis Sci. 2013. PMID: 23821205 Free PMC article.
-
Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina.J Neurosci. 2003 Jul 9;23(14):6063-73. doi: 10.1523/JNEUROSCI.23-14-06063.2003. J Neurosci. 2003. PMID: 12853425 Free PMC article.
-
Physiological characterization and functional heterogeneity of narrow-field mammalian amacrine cells.J Physiol. 2012 Jan 1;590(1):223-34. doi: 10.1113/jphysiol.2011.222141. Epub 2011 Nov 14. J Physiol. 2012. PMID: 22083601 Free PMC article.
Cited by
-
Generators of Pressure-Evoked Currents in Vertebrate Outer Retinal Neurons.Cells. 2021 May 22;10(6):1288. doi: 10.3390/cells10061288. Cells. 2021. PMID: 34067375 Free PMC article.
-
Dual-Cell Patch-Clamp Recording Revealed a Mechanism for a Ribbon Synapse to Process Both Digital and Analog Inputs and Outputs.Front Cell Neurosci. 2021 Oct 14;15:722533. doi: 10.3389/fncel.2021.722533. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34720878 Free PMC article.
-
Development of Retinal Amacrine Cells and Their Dendritic Stratification.Curr Ophthalmol Rep. 2014 Sep 1;2(3):100-106. doi: 10.1007/s40135-014-0048-2. Curr Ophthalmol Rep. 2014. PMID: 25170430 Free PMC article.
-
TRPV4 affects visual signals in photoreceptors and rod bipolar cells.Front Cell Neurosci. 2024 Jun 5;18:1404929. doi: 10.3389/fncel.2024.1404929. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38903773 Free PMC article.
-
Light-evoked S-nitrosylation in the retina.J Comp Neurol. 2015 Oct 1;523(14):2082-110. doi: 10.1002/cne.23780. Epub 2015 May 12. J Comp Neurol. 2015. PMID: 25823749 Free PMC article.
References
-
- Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12:155–165. - PubMed
-
- Akopian A, Witkovsky P. Calcium and retinal function. Mol Neurobiol. 2002;25:113–132. - PubMed
-
- Bugnon O, Schaad NC, Schorderet M. Nitric oxide modulates endogenous dopamine release in bovine retina. Neuroreport. 1994;5:401–404. - PubMed
-
- Chiou GC. Review: effects of nitric oxide on eye diseases and their treatment. J Ocul Pharmacol Ther. 2001;17:189–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

