Diffusion-weighted MRI for assessment of early cancer treatment response
- PMID: 20504274
- PMCID: PMC4003912
- DOI: 10.2174/138920110792246627
Diffusion-weighted MRI for assessment of early cancer treatment response
Abstract
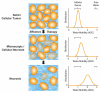
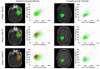
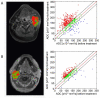
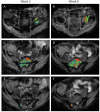
Recent clinical practice for the management for cancer patients has begun to change from a statistical "one-size fits all" approach to medicine to more individualized care. Pre-treatment biomarkers (i.e. genetically and histologically based) have a growing role in providing guidance related to the appropriate therapy and likelihood of response; they do not take into account heterogeneity within the tumor mass. Thus, a biomarker which could be utilized to measure actual tumor response early following treatment initiation would provide an important opportunity to evaluate treatment effects on an individual patient basis. Diffusion weighted magnetic resonance imaging (DW-MRI) offers the opportunity to monitor treatment-associated alterations in tumor microenvironment using quantification of changes in tumor water diffusion values as a surrogate imaging biomarker. Results obtained thus far using DW-MRI have shown that changes in tumor diffusion values can be detected early following treatment initiation which correlate with traditional outcome measures. Sensitive imaging biomarkers are providing for the first time a means of assessing 3 dimensional tumor response early in the treatment cycle. This review highlights the development of DW-MRI and its proposed usefulness in the clinical management of cancer patients. The utility of DW-MRI for assessing therapeutic-induced response is further evaluated on tumors residing in the brain, head and neck and bone.
Figures

Similar articles
-
Diffusion MRI in early cancer therapeutic response assessment.NMR Biomed. 2017 Mar;30(3):10.1002/nbm.3458. doi: 10.1002/nbm.3458. Epub 2016 Jan 15. NMR Biomed. 2017. PMID: 26773848 Free PMC article. Review.
-
Use of diffusion-weighted, intravoxel incoherent motion, and dynamic contrast-enhanced MR imaging in the assessment of response to radiotherapy of lytic bone metastases from breast cancer.Acad Radiol. 2014 Oct;21(10):1286-93. doi: 10.1016/j.acra.2014.05.021. Epub 2014 Aug 1. Acad Radiol. 2014. PMID: 25088834 Clinical Trial.
-
Diffusion weighted magnetic resonance imaging (DW-MRI) as a non-invasive, tissue cellularity marker to monitor cancer treatment response.BMC Cancer. 2020 Feb 19;20(1):134. doi: 10.1186/s12885-020-6617-x. BMC Cancer. 2020. PMID: 32075610 Free PMC article.
-
Predicting and monitoring cancer treatment response with diffusion-weighted MRI.J Magn Reson Imaging. 2010 Jul;32(1):2-16. doi: 10.1002/jmri.22167. J Magn Reson Imaging. 2010. PMID: 20575076 Free PMC article. Review.
-
Clinical utility of diffusion-weighted magnetic resonance imaging in prostate cancer.BJU Int. 2011 Dec;108(11):1716-22. doi: 10.1111/j.1464-410X.2011.10256.x. Epub 2011 Jun 1. BJU Int. 2011. PMID: 21631696 Review.
Cited by
-
MR Imaging Biomarkers to Monitor Early Response to Hypoxia-Activated Prodrug TH-302 in Pancreatic Cancer Xenografts.PLoS One. 2016 May 26;11(5):e0155289. doi: 10.1371/journal.pone.0155289. eCollection 2016. PLoS One. 2016. PMID: 27227903 Free PMC article.
-
DCE and DW-MRI monitoring of vascular disruption following VEGF-Trap treatment of a rat glioma model.NMR Biomed. 2012 Jul;25(7):935-42. doi: 10.1002/nbm.1814. Epub 2011 Dec 22. NMR Biomed. 2012. PMID: 22190279 Free PMC article.
-
Consensus recommendations on standardized magnetic resonance imaging protocols for multicenter canine brain tumor clinical trials.Vet Radiol Ultrasound. 2018 May;59(3):261-271. doi: 10.1111/vru.12608. Epub 2018 Mar 9. Vet Radiol Ultrasound. 2018. PMID: 29522650 Free PMC article.
-
Strategies to optimize radiotherapy based on biological responses of tumor and normal tissue.Exp Ther Med. 2012 Aug;4(2):175-180. doi: 10.3892/etm.2012.593. Epub 2012 May 30. Exp Ther Med. 2012. PMID: 22970024 Free PMC article.
-
PET/MR in oncology: an introduction with focus on MR and future perspectives for hybrid imaging.Am J Nucl Med Mol Imaging. 2012;2(4):458-74. Epub 2012 Oct 15. Am J Nucl Med Mol Imaging. 2012. PMID: 23145362 Free PMC article.
References
-
- Gehan EA, Schneiderman MA. Historical and methodological developments in clinical trials at the National Cancer Institute. Stat Med. 1990;9:871–880. discussion 903-876. - PubMed
-
- World Health Organization WHO handbook for reporting results of cancer treatment. Geneva, The Organization. 1979
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. - PubMed
-
- Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–3251. - PubMed
-
- Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–1628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

