Immunomodulatory properties of stem cells from human exfoliated deciduous teeth
- PMID: 20504286
- PMCID: PMC2873699
- DOI: 10.1186/scrt5
Immunomodulatory properties of stem cells from human exfoliated deciduous teeth
Abstract
Introduction: Stem cells from human exfoliated deciduous teeth (SHED) have been identified as a population of postnatal stem cells capable of differentiating into osteogenic and odontogenic cells, adipogenic cells, and neural cells. Herein we have characterized mesenchymal stem cell properties of SHED in comparison to human bone marrow mesenchymal stem cells (BMMSCs).
Methods: We used in vitro stem cell analysis approaches, including flow cytometry, inductive differentiation, telomerase activity, and Western blot analysis to assess multipotent differentiation of SHED and in vivo implantation to assess tissue regeneration of SHED. In addition, we utilized systemic SHED transplantation to treat systemic lupus erythematosus (SLE)-like MRL/lpr mice.
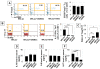
Results: We found that SHED are capable of differentiating into osteogenic and adipogenic cells, expressing mesenchymal surface molecules (STRO-1, CD146, SSEA4, CD73, CD105, and CD166), and activating multiple signaling pathways, including TGFbeta, ERK, Akt, Wnt, and PDGF. Recently, BMMSCs were shown to possess an immunomodulatory function that leads to successful therapies for immune diseases. We examined the immunomodulatory properties of SHED in comparison to BMMSCs and found that SHED had significant effects on inhibiting T helper 17 (Th17) cells in vitro. Moreover, we found that SHED transplantation is capable of effectively reversing SLE-associated disorders in MRL/lpr mice. At the cellular level, SHED transplantation elevated the ratio of regulatory T cells (Tregs) via Th17 cells.
Conclusions: These data suggest that SHED are an accessible and feasible mesenchymal stem cell source for treating immune disorders like SLE.
Figures


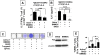

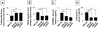
Similar articles
-
Transplantation of mesenchymal stem cells ameliorates secondary osteoporosis through interleukin-17-impaired functions of recipient bone marrow mesenchymal stem cells in MRL/lpr mice.Stem Cell Res Ther. 2015 May 27;6(1):104. doi: 10.1186/s13287-015-0091-4. Stem Cell Res Ther. 2015. PMID: 26012584 Free PMC article.
-
Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine.PLoS One. 2012;7(12):e51777. doi: 10.1371/journal.pone.0051777. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23251621 Free PMC article.
-
Targeting of Deciduous Tooth Pulp Stem Cell-Derived Extracellular Vesicles on Telomerase-Mediated Stem Cell Niche and Immune Regulation in Systemic Lupus Erythematosus.J Immunol. 2021 Jun 15;206(12):3053-3063. doi: 10.4049/jimmunol.2001312. Epub 2021 Jun 2. J Immunol. 2021. PMID: 34078710
-
A New Target of Dental Pulp-Derived Stem Cell-Based Therapy on Recipient Bone Marrow Niche in Systemic Lupus Erythematosus.Int J Mol Sci. 2022 Mar 23;23(7):3479. doi: 10.3390/ijms23073479. Int J Mol Sci. 2022. PMID: 35408840 Free PMC article. Review.
-
Stem Cells from Human Exfoliated Deciduous Teeth: A Concise Review.Curr Stem Cell Res Ther. 2020;15(1):61-76. doi: 10.2174/1574888X14666191018122109. Curr Stem Cell Res Ther. 2020. PMID: 31648649 Review.
Cited by
-
Impact of stem cells in craniofacial regenerative medicine.Front Physiol. 2012 Jun 21;3:188. doi: 10.3389/fphys.2012.00188. eCollection 2012. Front Physiol. 2012. PMID: 22737127 Free PMC article.
-
Toward personalized cell therapies by using stem cells: seven relevant topics for safety and success in stem cell therapy.J Biomed Biotechnol. 2012;2012:758102. doi: 10.1155/2012/758102. Epub 2012 Nov 20. J Biomed Biotechnol. 2012. PMID: 23226945 Free PMC article. Review.
-
Establishing methods for isolation of stem cells from human exfoliated deciduous from carious deciduous teeth.Interv Med Appl Sci. 2018 Mar;10(1):33-37. doi: 10.1556/1646.10.2018.06. Interv Med Appl Sci. 2018. PMID: 30363356 Free PMC article.
-
Animal Models for Stem Cell-Based Pulp Regeneration: Foundation for Human Clinical Applications.Tissue Eng Part B Rev. 2019 Apr;25(2):100-113. doi: 10.1089/ten.TEB.2018.0194. Epub 2019 Jan 9. Tissue Eng Part B Rev. 2019. PMID: 30284967 Free PMC article. Review.
-
Pulp-Dentin Tissue Healing Response: A Discussion of Current Biomedical Approaches.J Clin Med. 2020 Feb 5;9(2):434. doi: 10.3390/jcm9020434. J Clin Med. 2020. PMID: 32033375 Free PMC article. Review.
References
-
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. - DOI - PubMed
-
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

