Complement regulator Factor H mediates a two-step uptake of Streptococcus pneumoniae by human cells
- PMID: 20504767
- PMCID: PMC2906339
- DOI: 10.1074/jbc.M110.142703
Complement regulator Factor H mediates a two-step uptake of Streptococcus pneumoniae by human cells
Abstract
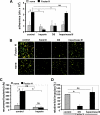
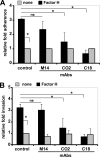
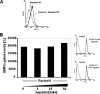
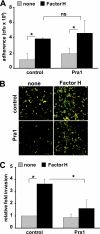
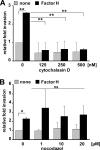
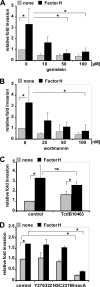
Streptococcus pneumoniae, a human pathogen, recruits complement regulator factor H to its bacterial cell surface. The bacterial PspC protein binds Factor H via short consensus repeats (SCR) 8-11 and SCR19-20. In this study, we define how bacterially bound Factor H promotes pneumococcal adherence to and uptake by epithelial cells or human polymorphonuclear leukocytes (PMNs) via a two-step process. First, pneumococcal adherence to epithelial cells was significantly reduced by heparin and dermatan sulfate. However, none of the glycosaminoglycans affected binding of Factor H to pneumococci. Adherence of pneumococci to human epithelial cells was inhibited by monoclonal antibodies recognizing SCR19-20 of Factor H suggesting that the C-terminal glycosaminoglycan-binding region of Factor H mediates the contact between pneumococci and human cells. Blocking of the integrin CR3 receptor, i.e. CD11b and CD18, of PMNs or CR3-expressing epithelial cells reduced significantly the interaction of pneumococci with both cell types. Similarly, an additional CR3 ligand, Pra1, derived from Candida albicans, blocked the interaction of pneumococci with PMNs. Strikingly, Pra1 inhibited also pneumococcal uptake by lung epithelial cells but not adherence. In addition, invasion of Factor H-coated pneumococci required the dynamics of host-cell actin microfilaments and was affected by inhibitors of protein-tyrosine kinases and phosphatidylinositol 3-kinase. In conclusion, pneumococcal entry into host cells via Factor H is based on a two-step mechanism. The first and initial contact of Factor H-coated pneumococci is mediated by glycosaminoglycans expressed on the surface of human cells, and the second step, pneumococcal uptake, is integrin-mediated and depends on host signaling molecules such as phosphatidylinositol 3-kinase.
Figures



References
-
- Austrian R. (1986) J. Antimicrob. Chemother. 18, Suppl. A, 35–45 - PubMed
-
- Cartwright K. (2002) Eur. J. Pediatr. 161, 188–195 - PubMed
-
- Hammerschmidt S. (2006) Curr. Opin. Microbiol. 9, 12–20 - PubMed
-
- Musher D. M. (1992) Clin. Infect. Dis. 14, 801–807 - PubMed
-
- Bergmann S., Hammerschmidt S. (2006) Microbiology 152, 295–303 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

