Electrophoretic deposition of biomaterials
- PMID: 20504802
- PMCID: PMC2952181
- DOI: 10.1098/rsif.2010.0156.focus
Electrophoretic deposition of biomaterials
Abstract
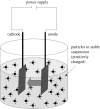
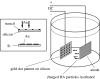
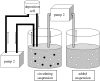
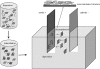
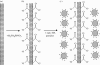
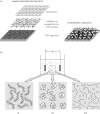
Electrophoretic deposition (EPD) is attracting increasing attention as an effective technique for the processing of biomaterials, specifically bioactive coatings and biomedical nanostructures. The well-known advantages of EPD for the production of a wide range of microstructures and nanostructures as well as unique and complex material combinations are being exploited, starting from well-dispersed suspensions of biomaterials in particulate form (microsized and nanoscale particles, nanotubes, nanoplatelets). EPD of biological entities such as enzymes, bacteria and cells is also being investigated. The review presents a comprehensive summary and discussion of relevant recent work on EPD describing the specific application of the technique in the processing of several biomaterials, focusing on (i) conventional bioactive (inorganic) coatings, e.g. hydroxyapatite or bioactive glass coatings on orthopaedic implants, and (ii) biomedical nanostructures, including biopolymer-ceramic nanocomposites, carbon nanotube coatings, tissue engineering scaffolds, deposition of proteins and other biological entities for sensors and advanced functional coatings. It is the intention to inform the reader on how EPD has become an important tool in advanced biomaterials processing, as a convenient alternative to conventional methods, and to present the potential of the technique to manipulate and control the deposition of a range of nanomaterials of interest in the biomedical and biotechnology fields.
Figures









References
-
- Affoune A. M., Prasad B. L. V., Sato H., Enoki T. 2001. Electrophoretic deposition of nanosized diamond particles. Langmuir 17, 547–551. (10.1021/la001317f) - DOI
-
- Ahmad Z., Nangrejo M., Edirisinghe M., Stride E., Colombo P., Zhang H. B. 2009. Engineering a material for biomedical applications with electric field assisted processing. Appl. Phys. A. Mater. Sci. Process. 97, 31–37.
-
- Akasaka T., Watari F., Sato Y., Tohji K. 2006. Apatite formation on carbon nanotubes. Mater. Sci. Eng. C 26, 675–678. (10.1016/j.msec.2005.03.009) - DOI
-
- Albayrak O., El-Atwani O., Altintas S. 2008. Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf. Coat. Technol. 202, 2482–2487. (10.1016/j.surfcoat.2007.09.031) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

