Customized Ca-P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor
- PMID: 20504805
- PMCID: PMC3024573
- DOI: 10.1098/rsif.2010.0127.focus
Customized Ca-P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor
Abstract
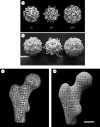
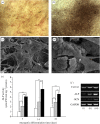
Integrating an advanced manufacturing technique, nanocomposite material and controlled delivery of growth factor to form multifunctional tissue engineering scaffolds was investigated in this study. Based on calcium phosphate (Ca-P)/poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) nanocomposite microspheres, three-dimensional Ca-P/PHBV nanocomposite scaffolds with customized architecture, controlled porosity and totally interconnected porous structure were successfully fabricated using selective laser sintering (SLS), one of the rapid prototyping technologies. The cytocompatibility of sintered Ca-P/PHBV nanocomposite scaffolds, as well as PHBV polymer scaffolds, was studied. For surface modification of nanocomposite scaffolds, gelatin was firstly physically entrapped onto the scaffold surface and heparin was subsequently immobilized on entrapped gelatin. The surface-modification improved the wettability of scaffolds and provided specific binding site between conjugated heparin and the growth factor recombinant human bone morphogenetic protein-2 (rhBMP-2). The surface-modified Ca-P/PHBV nanocomposite scaffolds loaded with rhBMP-2 significantly enhanced the alkaline phosphatase activity and osteogenic differentiation markers in gene expression of C3H10T1/2 mesenchymal stem cells. Together with osteoconductive nanocomposite material and controlled growth factor delivery strategies, the use of SLS technique to form complex scaffolds will provide a promising route towards individualized bone tissue regeneration.
Figures



Similar articles
-
Optimized fabrication of Ca-P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering.Biofabrication. 2011 Mar;3(1):015001. doi: 10.1088/1758-5082/3/1/015001. Epub 2011 Jan 18. Biofabrication. 2011. PMID: 21245522
-
Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering.Biofabrication. 2017 Jun 7;9(2):025031. doi: 10.1088/1758-5090/aa71c9. Biofabrication. 2017. PMID: 28589918
-
Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering.Acta Biomater. 2010 Dec;6(12):4495-505. doi: 10.1016/j.actbio.2010.06.024. Epub 2010 Jun 30. Acta Biomater. 2010. PMID: 20601244
-
Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication.Int J Mol Sci. 2023 Jul 19;24(14):11674. doi: 10.3390/ijms241411674. Int J Mol Sci. 2023. PMID: 37511432 Free PMC article. Review.
-
A review on the effect of nanocomposite scaffolds reinforced with magnetic nanoparticles in osteogenesis and healing of bone injuries.Stem Cell Res Ther. 2023 Aug 4;14(1):194. doi: 10.1186/s13287-023-03426-0. Stem Cell Res Ther. 2023. PMID: 37542279 Free PMC article. Review.
Cited by
-
Polysaccharide-based nanocomposites and their applications.Carbohydr Res. 2015 Mar 20;405:23-32. doi: 10.1016/j.carres.2014.07.016. Epub 2014 Jul 30. Carbohydr Res. 2015. PMID: 25498200 Free PMC article. Review.
-
Effect of Local Sustainable Release of BMP2-VEGF from Nano-Cellulose Loaded in Sponge Biphasic Calcium Phosphate on Bone Regeneration.Tissue Eng Part A. 2015 Jun;21(11-12):1822-36. doi: 10.1089/ten.TEA.2014.0497. Epub 2015 Apr 29. Tissue Eng Part A. 2015. PMID: 25808925 Free PMC article.
-
Dual-source dual-power electrospinning and characteristics of multifunctional scaffolds for bone tissue engineering.J Mater Sci Mater Med. 2012 Oct;23(10):2381-97. doi: 10.1007/s10856-012-4669-4. Epub 2012 May 17. J Mater Sci Mater Med. 2012. PMID: 22592965 Free PMC article.
-
Recent advances in 3D printing of biomaterials.J Biol Eng. 2015 Mar 1;9:4. doi: 10.1186/s13036-015-0001-4. eCollection 2015. J Biol Eng. 2015. PMID: 25866560 Free PMC article.
-
Scaling the heights--challenges in medical materials.J R Soc Interface. 2010 Oct 6;7 Suppl 5(Suppl 5):S501-2. doi: 10.1098/rsif.2010.0363.focus. Epub 2010 Jul 28. J R Soc Interface. 2010. PMID: 20667845 Free PMC article. No abstract available.
References
-
- Ang T. H., Sultana F. S. A., Hutmacher D. W., Wong Y. S., Fuh J. Y. H., Mo X. M., Loh H. T., Burdet E., Teoh S. H. 2002. Fabrication of 3D chitosan-hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 20, 35–42.
-
- Cao Y., Croll T. I., O'Connor A. J., Stevens G. W., Cooper-White J. J. 2006. Systematic selection of solvents for the fabrication of 3D combined macro- and microporous polymeric scaffolds for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 17, 369–402. (10.1163/156856206776374142) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

