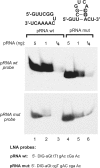
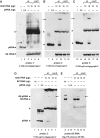
Northern blot detection of endogenous small RNAs (approximately14 nt) in bacterial total RNA extracts
- PMID: 20504856
- PMCID: PMC2919735
- DOI: 10.1093/nar/gkq437
Northern blot detection of endogenous small RNAs (approximately14 nt) in bacterial total RNA extracts
Abstract
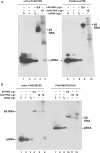
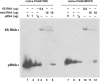
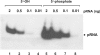
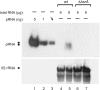
Here we describe a northern blot procedure that allows the detection of endogenous RNAs as small as approximately 14 nt in total RNA extracts from bacteria. RNAs that small and as part of total bacterial RNA extracts usually escape detection by northern blotting. The approach combines LNA probes 5'-digoxigenin-endlabeled for non-radioactive probe detection with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide-mediated chemical crosslinking of RNAs to nylon membranes, and necessitates the use of native PAGE either with the TBE or MOPS buffer system.
Figures


References
-
- Várallyay E, Burgyán J, Havelda Z. Detection of microRNAs by northern blot analyses using LNA probes. Methods. 2007;43:140–145. - PubMed
-
- Berezikov E, Thuemmler F, van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RHA. Diversity of microRNAs in human and chimpanzee brain. Nat. Genet. 2006;38:1375–1377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

