A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose
- PMID: 20504927
- PMCID: PMC2897621
- DOI: 10.1128/JVI.00200-10
A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose
Abstract
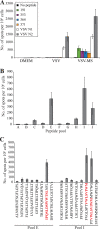
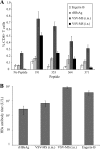
As one of the world's most common infectious diseases, hepatitis B virus (HBV) is a serious worldwide public health problem, with HBV-associated liver disease accounting for more than half a million deaths each year. Although there is an effective prophylactic vaccine currently available to prevent infection, it has a number of characteristics that are suboptimal: multiple doses are needed to induce long-lasting immunity, immunity declines over time, it does not elicit protection in some individuals, and it is not effective therapeutically. We produced a recombinant vesicular stomatitis virus (VSV)-based vaccine vector expressing the HBV middle envelope surface protein (MS) and found that this vector was able to efficiently generate a strong HBs-specific antibody response following a single immunization in mice. A single immunization with the VSV-MS vector also induced robust CD8 T-cell activation. The CD8 T-cell response was greater in magnitude and broader in specificity than the response generated by a vaccinia virus-based vaccine vector or by recombinant protein immunization. Furthermore, a single VSV-MS immunization provided protection against virus challenge in mice. Given the similar antibody titers and superior T-cell responses elicited from a single immunization, a VSV-based HBV vaccine may have advantages over the current recombinant protein vaccine.
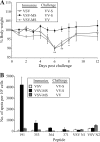
Figures


Similar articles
-
Virus-Like Vesicle-Based Therapeutic Vaccine Vectors for Chronic Hepatitis B Virus Infection.J Virol. 2015 Oct;89(20):10407-15. doi: 10.1128/JVI.01184-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246574 Free PMC article.
-
Heterologous prime-boost immunization with vesiculovirus-based vectors expressing HBV Core antigen induces CD8+ T cell responses in naïve and persistently infected mice and protects from challenge.Antiviral Res. 2019 Aug;168:156-167. doi: 10.1016/j.antiviral.2019.05.014. Epub 2019 May 30. Antiviral Res. 2019. PMID: 31153968 Free PMC article.
-
A Highly Attenuated Vesicular Stomatitis Virus-Based Vaccine Platform Controls Hepatitis B Virus Replication in Mouse Models of Hepatitis B.J Virol. 2019 Feb 19;93(5):e01586-18. doi: 10.1128/JVI.01586-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541859 Free PMC article.
-
Hepatitis B vaccine effectiveness in the face of global HBV genotype diversity.Expert Rev Vaccines. 2011 Dec;10(12):1709-15. doi: 10.1586/erv.11.151. Expert Rev Vaccines. 2011. PMID: 22085174 Review.
-
DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response.Ann N Y Acad Sci. 1995 Nov 27;772:64-76. doi: 10.1111/j.1749-6632.1995.tb44732.x. Ann N Y Acad Sci. 1995. PMID: 8546414 Review.
Cited by
-
Virus-Like Vesicle-Based Therapeutic Vaccine Vectors for Chronic Hepatitis B Virus Infection.J Virol. 2015 Oct;89(20):10407-15. doi: 10.1128/JVI.01184-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246574 Free PMC article.
-
Inhibition of hepatitis B virus (HBV) gene expression and replication by HBx gene silencing in a hydrodynamic injection mouse model with a new clone of HBV genotype B.Virol J. 2013 Jun 28;10:214. doi: 10.1186/1743-422X-10-214. Virol J. 2013. PMID: 23805945 Free PMC article.
-
Immunovirotherapy Based on Recombinant Vesicular Stomatitis Virus: Where Are We?Front Immunol. 2022 Jun 28;13:898631. doi: 10.3389/fimmu.2022.898631. eCollection 2022. Front Immunol. 2022. PMID: 35837384 Free PMC article. Review.
-
Mutations in the glycoprotein of vesicular stomatitis virus affect cytopathogenicity: potential for oncolytic virotherapy.J Virol. 2011 Jul;85(13):6513-20. doi: 10.1128/JVI.02484-10. Epub 2011 May 11. J Virol. 2011. PMID: 21561919 Free PMC article.
-
Vesicular Stomatitis Virus-Vectored Multi-Antigen Tuberculosis Vaccine Limits Bacterial Proliferation in Mice following a Single Intranasal Dose.Front Cell Infect Microbiol. 2017 Feb 7;7:34. doi: 10.3389/fcimb.2017.00034. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28224119 Free PMC article.
References
-
- Barnaba, V., A. Franco, A. Alberti, C. Balsano, R. Benvenuto, and F. Balsano. 1989. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J. Immunol. 143:2650-2655. - PubMed
-
- Bertoletti, A., and A. Gehring. 2009. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev. Gastroenterol. Hepatol. 3:561-569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

