Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells
- PMID: 20504948
- PMCID: PMC2924605
- DOI: 10.1189/jlb.1209822
Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells
Abstract
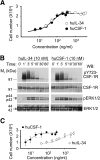
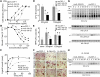
CSF-1 is broadly expressed and regulates macrophage and osteoclast development. The action and expression of IL-34, a novel CSF-1R ligand, were investigated in the mouse. As expected, huIL-34 stimulated macrophage proliferation via the huCSF-1R, equivalently to huCSF-1, but was much less active at stimulating mouse macrophage proliferation than huCSF-1. Like muCSF-1, muIL-34 and a muIL-34 isoform lacking Q81 stimulated mouse macrophage proliferation, CSF-1R tyrosine phosphorylation, and signaling and synergized with other cytokines to generate macrophages and osteoclasts from cultured progenitors. However, they respectively possessed twofold and fivefold lower affinities for the CSF-1R and correspondingly, lower activities than muCSF-1. Furthermore, muIL-34, when transgenically expressed in a CSF-1-dependent manner in vivo, rescued the bone, osteoclast, tissue macrophage, and fertility defects of Csf1(op)/(op) mice, suggesting similar regulation of CSF-1R-expressing cells by IL-34 and CSF-1. Whole-mount IL34 in situ hybridization and CSF-1 reporter expression revealed that IL34 mRNA was strongly expressed in the embryonic brain at E11.5, prior to the expression of Csf1 mRNA. QRT-PCR revealed that compared with Csf1 mRNA, IL34 mRNA levels were lower in pregnant uterus and in cultured osteoblasts, higher in most regions of the brain and heart, and not compensatorily increased in Csf1(op/op) mouse tissues. Thus, the different spatiotemporal expression of IL-34 and CSF-1 allows for complementary activation of the CSF-1R in developing and adult tissues.
Figures





References
-
- Stanley E R, Chen D M, Lin H-S. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978;274:168–170. - PubMed
-
- Tushinski R J, Oliver I T, Guilbert L J, Tynan P W, Warner J R, Stanley E R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. - PubMed
-
- Cecchini M G, Dominguez M G, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard J W, Stanley E R. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. - PubMed
-
- Pixley F J, Stanley E R. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

