Auxin perception--structural insights
- PMID: 20504967
- PMCID: PMC2890193
- DOI: 10.1101/cshperspect.a005546
Auxin perception--structural insights
Abstract
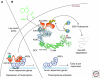
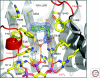
The identity of the auxin receptor(s) and the mechanism of auxin perception has been a subject of intense interest since the discovery of auxin almost a century ago. The development of genetic approaches to the study of plant hormone signaling led to the discovery that auxin acts by promoting degradation of transcriptional repressors called Aux/IAA proteins. This process requires a ubiquitin protein ligase (E3) called SCF(TIR1) and related SCF complexes. Surprisingly, auxin works by directly binding to TIR1, the F-box protein subunit of this SCF. Structural studies demonstrate that auxin acts like a "molecular glue," to stabilize the interaction between TIR1 and the Aux/IAA substrate. These exciting results solve an old problem in plant biology and reveal new mechanisms for E3 regulation and hormone perception.
Figures

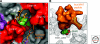
References
-
- Bates PW, Vierstra RD 1999. UPL1 and 2, two 450 kDa ubiquitin-protein ligases from Arabidopsis thaliana related to the HECT-domain protein family. Plant J 20:183–195 - PubMed
-
- Baumeister W, Walz J, Zühl F, Seemüller E 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367–380 - PubMed
-
- Cardozo T, Pagano M 2004. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol 5:739–751 - PubMed
-
- Chapman EJ, Estelle M 2009. Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 43:265–285 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
