The MeCP2/YY1 interaction regulates ANT1 expression at 4q35: novel hints for Rett syndrome pathogenesis
- PMID: 20504995
- PMCID: PMC2908467
- DOI: 10.1093/hmg/ddq214
The MeCP2/YY1 interaction regulates ANT1 expression at 4q35: novel hints for Rett syndrome pathogenesis
Abstract
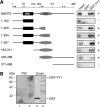
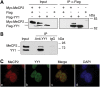
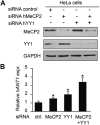
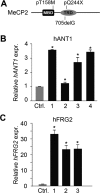
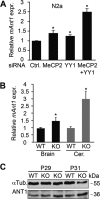
Rett syndrome is a severe neurodevelopmental disorder mainly caused by mutations in the transcriptional regulator MeCP2. Although there is no effective therapy for Rett syndrome, the recently discovered disease reversibility in mice suggests that there are therapeutic possibilities. Identification of MeCP2 targets or modifiers of the phenotype can facilitate the design of curative strategies. To identify possible novel MeCP2 interactors, we exploited a bioinformatic approach and selected Ying Yang 1 (YY1) as an interesting candidate. We demonstrate that MeCP2 interacts in vitro and in vivo with YY1, a ubiquitous zinc-finger epigenetic factor regulating the expression of several genes. We show that MeCP2 cooperates with YY1 in repressing the ANT1 gene encoding a mitochondrial adenine nucleotide translocase. Importantly, ANT1 mRNA levels are increased in human and mouse cell lines devoid of MeCP2, in Rett patient fibroblasts and in the brain of Mecp2-null mice. We further demonstrate that ANT1 protein levels are upregulated in Mecp2-null mice. Finally, the identified MeCP2-YY1 interaction, together with the well-known involvement of YY1 in the regulation of D4Z4-associated genes at 4q35, led us to discover the anomalous depression of FRG2, a subtelomeric gene of unknown function, in Rett fibroblasts. Collectively, our data indicate that mutations in MeCP2 might cause the aberrant overexpression of genes located at a specific locus, thus providing new candidates for the pathogenesis of Rett syndrome. As both ANT1 mutations and overexpression have been associated with human diseases, we consider it highly relevant to address the consequences of ANT1 deregulation in Rett syndrome.
Figures

References
-
- Jones P.L., Veenstra G.J., Wade P.A., Vermaak D., Kass S.U., Landsberger N., Strouboulis J., Wolffe A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998;19:187–191. doi:10.1038/561. - DOI - PubMed
-
- Nan X., Ng H.H., Johnson C.A., Laherty C.D., Turner B.M., Eisenman R.N., Bird A. Transcriptional repression by the methyl-CpG binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. - PubMed
-
- Fuks F., Hurd P.J., Deplus R., Kouzarides T. The methyl-CpG binding protein MeCP2 links DNA methylation to histone methylation. Nucleic Acids Res. 2003;31:2305–2312. doi:10.1093/nar/gkg332. - DOI - PMC - PubMed
-
- Nan X., Hou J., Maclean A., Nasir J., Lafuente M.J., Shu X., Kriaucionis S., Bird A. Interaction between chromatic proteins MECP2 and ATRX is disrupted by mutations that cause inherited mental retardation. Proc. Natl Acad. Sci. USA. 2007;104:2709–2714. doi:10.1073/pnas.0608056104. - DOI - PMC - PubMed
-
- Chahrour M., Jung S.Y., Shaw C., Zhou X., Wong S.T., Qin J., Zoghbi H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi:10.1126/science.1153252. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

