Biodegradation of resin-dentin interfaces increases bacterial microleakage
- PMID: 20505047
- PMCID: PMC3318074
- DOI: 10.1177/0022034510372885
Biodegradation of resin-dentin interfaces increases bacterial microleakage
Abstract
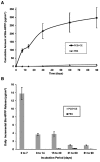
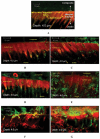
Bis-GMA-containing resin composites and adhesives undergo biodegradation by human-saliva-derived esterases, yielding Bis-hydroxy-propoxy-phenyl-propane (Bis-HPPP). The hypothesis of this study is that the exposure of dental restorations to saliva-like esterase activities accelerates marginal bacterial microleakage. Resin composites (Scotchbond, Z250, 3M) bonded to human dentin were incubated in either buffer or dual-esterase media (pseudocholinesterase/cholesterol-esterase; PCE+CE), with activity levels simulating those of human saliva, for up to 90 days. Incubation solutions were analyzed for Bis-HPPP by high-performance liquid chromatography. Post-incubation, specimens were suspended in a chemostat-based biofilm fermentor cultivating Streptococcus mutans NG8, a primary species associated with dental caries, for 7 days. Bacterial microleakage was assessed by confocal laser scanning microscopy. Bis-HPPP production and depth and spatial volume of bacterial cell penetration within the interface increased with incubation time and were higher for 30- and 90-day PCE+CE vs. buffer-incubated groups, suggesting that biodegradation can contribute to the formation of recurrent decay.
Figures


References
-
- Bouillaguet S. (2004). Biological risks of resin-based materials to the dentin-pulp complex. Crit Rev Oral Biol Med 15:47-60 - PubMed
-
- Donmez N, Belli S, Pashley DH, Tay FR. (2005). Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res 84:355-359 - PubMed
-
- Ferracane JL. (2006). Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater 22:221-222 - PubMed
-
- Finer Y, Santerre JP. (2003). Biodegradation of a dental composite by esterases: dependence on enzyme concentration and specificity. J Biomater Sci Polym Ed 14:837-849 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

