Scyl1 facilitates nuclear tRNA export in mammalian cells by acting at the nuclear pore complex
- PMID: 20505071
- PMCID: PMC2903676
- DOI: 10.1091/mbc.e10-03-0176
Scyl1 facilitates nuclear tRNA export in mammalian cells by acting at the nuclear pore complex
Abstract
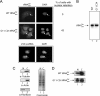
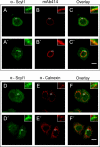
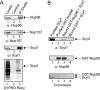
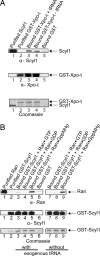
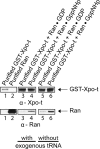
Scyl1 is an evolutionarily conserved N-terminal protein kinase-like domain protein that plays a role in COP1-mediated retrograde protein trafficking in mammalian cells. Furthermore, loss of Scyl1 function has been shown to result in neurodegenerative disorders in mice. Here, we report that Scyl1 is also a cytoplasmic component of the mammalian nuclear tRNA export machinery. Like exportin-t, overexpression of Scyl1 restored export of a nuclear export-defective serine amber suppressor tRNA mutant in COS-7 cells. Scyl1 binds tRNA saturably, and associates with the nuclear pore complex by interacting, in part, with Nup98. Scyl1 copurifies with the nuclear tRNA export receptors exportin-t and exportin-5, the RanGTPase, and the eukaryotic elongation factor eEF-1A, which transports aminoacyl-tRNAs to the ribosomes. Scyl1 interacts directly with exportin-t and RanGTP but not with eEF-1A or RanGDP in vitro. Moreover, exportin-t containing tRNA, Scyl1, and RanGTP form a quaternary complex in vitro. Biochemical characterization also suggests that the nuclear aminoacylation-dependent pathway is primarily responsible for tRNA export in mammalian cells. These findings together suggest that Scyl1 participates in the nuclear aminoacylation-dependent tRNA export pathway and may unload aminoacyl-tRNAs from the nuclear tRNA export receptor at the cytoplasmic side of the nuclear pore complex and channels them to eEF-1A.
Figures









References
-
- Arts G. J., Fornerod M., Mattaj I. W. Identification of a nuclear export receptor for tRNA. Curr. Biol. 1998a;8:305–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

