Unrestrained spindle elongation during recovery from spindle checkpoint activation in cdc15-2 cells results in mis-segregation of chromosomes
- PMID: 20505077
- PMCID: PMC2903668
- DOI: 10.1091/mbc.e09-07-0637
Unrestrained spindle elongation during recovery from spindle checkpoint activation in cdc15-2 cells results in mis-segregation of chromosomes
Abstract
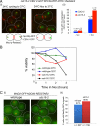
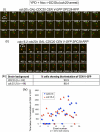
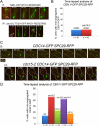
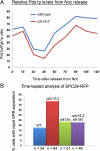
During normal metaphase in Saccharomyces cerevisiae, chromosomes are captured at the kinetochores by microtubules emanating from the spindle pole bodies at opposite poles of the dividing cell. The balance of forces between the cohesins holding the replicated chromosomes together and the pulling force from the microtubules at the kinetochores result in the biorientation of the sister chromatids before chromosome segregation. The absence of kinetochore-microtubule interactions or loss of cohesion between the sister chromatids triggers the spindle checkpoint which arrests cells in metaphase. We report here that an MEN mutant, cdc15-2, though competent in activating the spindle assembly checkpoint when exposed to Noc, mis-segregated chromosomes during recovery from spindle checkpoint activation. cdc15-2 cells arrested in Noc, although their Pds1p levels did not accumulate as well as in wild-type cells. Genetic analysis indicated that Pds1p levels are lower in a mad2Delta cdc15-2 and bub2Delta cdc15-2 double mutants compared with the single mutants. Chromosome mis-segregation in the mutant was due to premature spindle elongation in the presence of unattached chromosomes, likely through loss of proper control on spindle midzone protein Slk19p and kinesin protein, Cin8p. Our data indicate that a slower rate of transition through the cell division cycle can result in an inadequate level of Pds1p accumulation that can compromise recovery from spindle assembly checkpoint activation.
Figures





References
-
- Bardin A. J., Amon A. Men and sin: what's the difference? Nat. Rev. Mol. Cell Biol. 2001;2:815–826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

