The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia
- PMID: 20505092
- PMCID: PMC2909847
- DOI: 10.1523/JNEUROSCI.3582-09.2010
The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia
Abstract
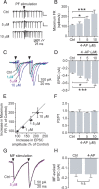
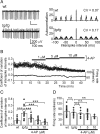
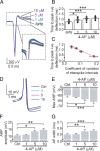
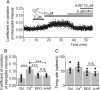
Episodic ataxia type 2 (EA2) is a hereditary cerebellar ataxia associated with mutations in the P/Q-type voltage-gated calcium (Ca(2+)) channels. Therapeutic approaches for treatment of EA2 are very limited. Presently, the potassium (K(+)) channel blocker 4-aminopyridine (4-AP) constitutes the most promising treatment, although its mechanism of action is not understood. Here we show that, in contrast to what is commonly believed, therapeutic concentrations of 4-AP do not increase the inhibitory drive of cerebellar Purkinje cells. Instead, 4-AP restores the severely diminished precision of pacemaking in Purkinje cells of EA2 mutant mice by prolonging the action potential and increasing the action potential afterhyperpolarization. Consistent with this mode of action, the therapeutic efficacy of 4-AP was comparable, and not additive, to chlorzoxazone, an activator of Ca(2+)-dependent K(+) channels that also restores the precision of Purkinje cell pacemaking. The likely target of 4-AP at the concentrations used are the K(v)1 family of K(+) channels, possibly the K(v)1.5 subtype. Because at higher concentrations 4-AP blocks a large array of K(+) channels and is a proconvulsant, use of selective K(v)1 channel blockers is likely to be a safer substitute for treatment of cerebellar ataxia.
Figures


References
-
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. - PMC - PubMed
-
- Bever CT, Jr, Young D, Anderson PA, Krumholz A, Conway K, Leslie J, Eddington N, Plaisance KI, Panitch HS, Dhib-Jalbut S. The effects of 4-aminopyridine in multiple sclerosis patients: results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trial. Neurology. 1994;44:1054–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
