Metabolic signatures of exercise in human plasma
- PMID: 20505214
- PMCID: PMC3010398
- DOI: 10.1126/scitranslmed.3001006
Metabolic signatures of exercise in human plasma
Abstract
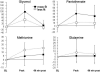
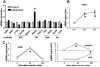
Exercise provides numerous salutary effects, but our understanding of how these occur is limited. To gain a clearer picture of exercise-induced metabolic responses, we have developed comprehensive plasma metabolite signatures by using mass spectrometry to measure >200 metabolites before and after exercise. We identified plasma indicators of glycogenolysis (glucose-6-phosphate), tricarboxylic acid cycle span 2 expansion (succinate, malate, and fumarate), and lipolysis (glycerol), as well as modulators of insulin sensitivity (niacinamide) and fatty acid oxidation (pantothenic acid). Metabolites that were highly correlated with fitness parameters were found in subjects undergoing acute exercise testing and marathon running and in 302 subjects from a longitudinal cohort study. Exercise-induced increases in glycerol were strongly related to fitness levels in normal individuals and were attenuated in subjects with myocardial ischemia. A combination of metabolites that increased in plasma in response to exercise (glycerol, niacinamide, glucose-6-phosphate, pantothenate, and succinate) up-regulated the expression of nur77, a transcriptional regulator of glucose utilization and lipid metabolism genes in skeletal muscle in vitro. Plasma metabolic profiles obtained during exercise provide signatures of exercise performance and cardiovascular disease susceptibility, in addition to highlighting molecular pathways that may modulate the salutary effects of exercise.
Figures




References
-
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Circulation. 2003;107:3109. - PubMed
-
- Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL, Jr., Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC., Jr. Circulation. 2002;106:1883. - PubMed
-
- McNeer JF, Margolis JR, Lee KL, Kisslo JA, Peter RH, Kong Y, Behar VS, Wallace AG, McCants CB, Rosati RA. Circulation. 1978;57:64. - PubMed
-
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. N Engl J Med. 2002;346:793. - PubMed
-
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. J Am Coll Cardiol. 1997;30:641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-HC-25195/HC/NHLBI NIH HHS/United States
- R01 HL072872/HL/NHLBI NIH HHS/United States
- R01DK081572/DK/NIDDK NIH HHS/United States
- R01 HL083141/HL/NHLBI NIH HHS/United States
- NS054052/NS/NINDS NIH HHS/United States
- R01 DK081572/DK/NIDDK NIH HHS/United States
- K23HL091106/HL/NHLBI NIH HHS/United States
- P50 HG004233/HG/NHGRI NIH HHS/United States
- HG0017115/HG/NHGRI NIH HHS/United States
- R01 HG003224/HG/NHGRI NIH HHS/United States
- K23 HL091106/HL/NHLBI NIH HHS/United States
- N01 HC025195/HL/NHLBI NIH HHS/United States
- U01HL083141/HL/NHLBI NIH HHS/United States
- HG003224/HG/NHGRI NIH HHS/United States
- R01 NS054052/NS/NINDS NIH HHS/United States
- HG004233/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

