Mining human antibody repertoires
- PMID: 20505349
- PMCID: PMC3180084
- DOI: 10.4161/mabs.12187
Mining human antibody repertoires
Abstract
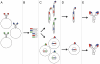
Human monoclonal antibodies (mAbs) have become drugs of choice for the management of an increasing number of human diseases. Human antibody repertoires provide a rich source for human mAbs. Here we review the characteristics of natural and non-natural human antibody repertoires and their mining with non-combinatorial and combinatorial strategies. In particular, we discuss the selection of human mAbs from naïve, immune, transgenic, and synthetic human antibody repertoires using methods based on hybridoma technology, clonal expansion of peripheral B cells, single-cell PCR, phage display, yeast display, and mammalian cell display. Our reliance on different strategies is shifting as we gain experience and refine methods to the efficient generation of human mAbs with superior pharmacokinetic and pharmacodynamic properties.
Figures

References
-
- Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3–10. - PubMed
-
- Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother. 2006;29:1–9. - PubMed
-
- Swann PG, Tolnay M, Muthukkumar S, Shapiro MA, Rellahan BL, Clouse KA. Considerations for the development of therapeutic monoclonal antibodies. Curr Opin Immunol. 2008;20:493–499. - PubMed
-
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
