A single ascending dose study of bapineuzumab in patients with Alzheimer disease
- PMID: 20505438
- PMCID: PMC3715117
- DOI: 10.1097/WAD.0b013e3181c53b00
A single ascending dose study of bapineuzumab in patients with Alzheimer disease
Abstract
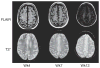
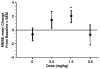
The safety, tolerability, and pharmacokinetics (PKs) of bapineuzumab (AAB-001), a humanized monoclonal antibody to amyloid beta, were evaluated in patients with mild-to-moderate Alzheimer disease in a phase 1, randomized, third-party unblinded, placebo-controlled, single ascending dose trial. Thirty patients received bapineuzumab infusion of 0.5, 1.5, or 5 mg/kg or placebo (6 active, 2 placebo for 0.5 and 1.5-mg/kg cohorts; 10 active, 4 placebo for 5.0-mg/kg cohort). Three patients in the highest dose cohort (5.0 mg/kg) developed magnetic resonance imaging abnormalities consistent with vasogenic edema, predominantly high signal abnormalities on fluid-attenuated inversion recovery sequences, all of which resolved over time. Plasma amyloid beta was elevated from baseline, peaking approximately 24 hours after infusion. PK analysis demonstrated a half-life of 21 to 26 days, supporting a 13-week dosing interval for bapineuzumab. This small, single-dose study demonstrated the safety profile and PK characteristics of bapineuzumab and was used to design later safety and efficacy trials.
Figures


References
-
- Goñi F, Sigurdsson EM. New directions towards safer and effective vaccines for Alzheimer’s disease. Curr Opin Mol Ther. 2005;7:17–23. - PubMed
-
- Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. - PubMed
-
- Morgan D, Diamond DM, Gottschall PE, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. - PubMed
-
- Janus C, Pearson J, McLaurin J, et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. - PubMed
-
- Orgogozo J-M, Gilman S, Dartigues J-F, et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

