Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria
- PMID: 20505657
- PMCID: PMC3065782
- DOI: 10.1038/ki.2010.137
Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria
Abstract
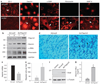
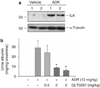
Proteinuria is a primary clinical symptom of a large number of glomerular diseases that progress to end-stage renal failure. Podocyte dysfunctions play a fundamental role in defective glomerular filtration in many common forms of proteinuric kidney disorders. Since binding of these cells to the basement membrane is mediated by integrins, we determined the role of integrin-linked kinase (ILK) in podocyte dysfunction and proteinuria. ILK expression was induced in mouse podocytes by various injurious stimuli known to cause proteinuria including TGF-beta1, adriamycin, puromycin, and high ambient glucose. Podocyte ILK was also found to be upregulated in human proteinuric glomerular diseases. Ectopic expression of ILK in podocytes decreased levels of the epithelial markers nephrin and ZO-1, induced mesenchymal markers such as desmin, fibronectin, matrix metalloproteinase-9 (MMP-9), and alpha-smooth muscle actin (alpha-SMA), promoted cell migration, and increased the paracellular albumin flux across podocyte monolayers. ILK also induced Snail, a key transcription factor mediating epithelial-mesenchymal transition (EMT). Blockade of ILK activity with a highly selective small molecule inhibitor reduced Snail induction and preserved podocyte phenotypes following TGF-beta1 or adriamycin stimulation. In vivo, this ILK inhibitor ameliorated albuminuria, repressed glomerular induction of MMP-9 and alpha-SMA, and preserved nephrin expression in murine adriamycin nephropathy. Our results show that upregulation of ILK is a convergent pathway leading to podocyte EMT, migration, and dysfunction. ILK may be an attractive target for therapeutic intervention of proteinuric kidney diseases.
Figures






References
-
- Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. - PubMed
-
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. - PubMed
-
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. - PubMed
-
- Tryggvason K, Wartiovaara J. Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens. 2001;10:543–549. - PubMed
-
- Reiser J, Kriz W, Kretzler M, et al. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

