Eef1a2 promotes cell growth, inhibits apoptosis and activates JAK/STAT and AKT signaling in mouse plasmacytomas
- PMID: 20505761
- PMCID: PMC2873962
- DOI: 10.1371/journal.pone.0010755
Eef1a2 promotes cell growth, inhibits apoptosis and activates JAK/STAT and AKT signaling in mouse plasmacytomas
Abstract
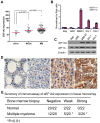
Background: The canonical function of EEF1A2, normally expressed only in muscle, brain, and heart, is in translational elongation, but recent studies suggest a non-canonical function as a proto-oncogene that is overexpressed in a variety of solid tumors including breast and ovary. Transcriptional profiling of a spectrum of primary mouse B cell lineage neoplasms showed that transcripts encoding EEF1A2 were uniquely overexpressed in plasmacytomas (PCT), tumors of mature plasma cells. Cases of human multiple myeloma expressed significantly higher levels of EEF1A2 transcripts than normal bone marrow plasma cells. High-level expression was also a feature of a subset of cell lines developed from mouse PCT and from the human MM.
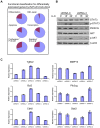
Methodology/principal findings: Heightened expression of EEF1A2 was not associated with increased copy number or coding sequence mutations. shRNA-mediated knockdown of Eef1a2 transcripts and protein was associated with growth inhibition due to delayed G1-S progression, and effects on apoptosis that were seen only under serum-starved conditions. Transcriptional profiles and western blot analyses of knockdown cells revealed impaired JAK/STAT and PI3K/AKT signaling suggesting their contributions to EEF1A2-mediated effects on PCT induction or progression.
Conclusions/significance: EEF1A2 may play contribute to the induction or progression of some PCT and a small percentage of MM. Eef1a2 could also prove to be a useful new marker for a subset of MM and, ultimately, a possible target for therapy.
Conflict of interest statement
Figures



References
-
- Li R, Wang H, Bekele BN, Yin Z, Caraway NP, et al. Identification of putative oncogenes in lung adenocarcinoma by a comprehensive functional genomic approach. Oncogene. 2006;25:2628–2635. - PubMed
-
- Kulkarni G, Turbin DA, Amiri A, Jeganathan S, Andrade-Navarro MA, et al. Expression of protein elongation factor eEF1A2 predicts favorable outcome in breast cancer. Breast Cancer Res Treat. 2007;102:31–41. - PubMed
-
- Grassi G, Scaggiante B, Farra R, Dapas B, Agostini F, et al. The expression levels of the translational factors eEF1A 1/2 correlate with cell growth but not apoptosis in hepatocellular carcinoma cell lines with different differentiation grade. Biochimie. 2007;89:1544–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

