Toll-like receptor 4 promoter polymorphisms: common TLR4 variants may protect against severe urinary tract infection
- PMID: 20505764
- PMCID: PMC2873976
- DOI: 10.1371/journal.pone.0010734
Toll-like receptor 4 promoter polymorphisms: common TLR4 variants may protect against severe urinary tract infection
Abstract
Background: Polymorphisms affecting Toll-like receptor (TLR) structure appear to be rare, as would be expected due to their essential coordinator role in innate immunity. Here, we assess variation in TLR4 expression, rather than structure, as a mechanism to diversify innate immune responses.
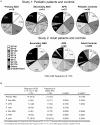
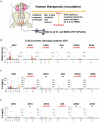
Methodology/principal findings: We sequenced the TLR4 promoter (4,3 kb) in Swedish blood donors. Since TLR4 plays a vital role in susceptibility to urinary tract infection (UTI), promoter sequences were obtained from children with mild or severe disease. We performed a case-control study of pediatric patients with asymptomatic bacteriuria (ABU) or those prone to recurrent acute pyelonephritis (APN). Promoter activity of the single SNPs or multiple allelic changes corresponding to the genotype patterns (GPs) was tested. We then conducted a replication study in an independent cohort of adult patients with a history of childhood APN. Last, in vivo effects of the different GPs were examined after therapeutic intravesical inoculation of 19 patients with Escherichia coli 83972. We identified in total eight TLR4 promoter sequence variants in the Swedish control population, forming 19 haplotypes and 29 genotype patterns, some with effects on promoter activity. Compared to symptomatic patients and healthy controls, ABU patients had fewer genotype patterns, and their promoter sequence variants reduced TLR4 expression in response to infection. The ABU associated GPs also reduced innate immune responses in patients who were subjected to therapeutic urinary E. coli tract inoculation.
Conclusions: The results suggest that genetic variation in the TLR4 promoter may be an essential, largely overlooked mechanism to influence TLR4 expression and UTI susceptibility.
Conflict of interest statement
Figures




Comment in
-
Genetics: TLR4 promoter variants influence response to UTI.Nat Rev Urol. 2010 Jul;7(7):365. doi: 10.1038/nrurol.2010.94. Nat Rev Urol. 2010. PMID: 20665938 No abstract available.
Similar articles
-
Genetic control of the variable innate immune response to asymptomatic bacteriuria.PLoS One. 2011;6(11):e28289. doi: 10.1371/journal.pone.0028289. Epub 2011 Nov 28. PLoS One. 2011. PMID: 22140570 Free PMC article.
-
Reduced toll-like receptor 4 expression in children with asymptomatic bacteriuria.J Infect Dis. 2007 Aug 1;196(3):475-84. doi: 10.1086/518893. Epub 2007 Jun 21. J Infect Dis. 2007. PMID: 17597463
-
Testing an association between TLR4 and CXCR1 gene polymorphisms with susceptibility to urinary tract infection in type 2 diabetes in north Indian population.Gene. 2018 Jan 30;641:196-202. doi: 10.1016/j.gene.2017.10.060. Epub 2017 Oct 21. Gene. 2018. PMID: 29066305
-
Innate immunity and genetic determinants of urinary tract infection susceptibility.Curr Opin Infect Dis. 2015 Feb;28(1):88-96. doi: 10.1097/QCO.0000000000000127. Curr Opin Infect Dis. 2015. PMID: 25539411 Free PMC article. Review.
-
TLR- and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections.Eur J Clin Invest. 2008 Oct;38 Suppl 2:12-20. doi: 10.1111/j.1365-2362.2008.02004.x. Eur J Clin Invest. 2008. PMID: 18826477 Review.
Cited by
-
Toll-like receptor signaling in primary immune deficiencies.Ann N Y Acad Sci. 2015 Nov;1356(1):1-21. doi: 10.1111/nyas.12763. Epub 2015 Apr 30. Ann N Y Acad Sci. 2015. PMID: 25930993 Free PMC article. Review.
-
Host imprints on bacterial genomes--rapid, divergent evolution in individual patients.PLoS Pathog. 2010 Aug 26;6(8):e1001078. doi: 10.1371/journal.ppat.1001078. PLoS Pathog. 2010. PMID: 20865122 Free PMC article.
-
The immune response to infection in the bladder.Nat Rev Urol. 2020 Aug;17(8):439-458. doi: 10.1038/s41585-020-0350-8. Epub 2020 Jul 13. Nat Rev Urol. 2020. PMID: 32661333 Review.
-
TLR4 genetic variation is associated with inflammatory responses in Gram-positive sepsis.Clin Microbiol Infect. 2017 Jan;23(1):47.e1-47.e10. doi: 10.1016/j.cmi.2016.08.028. Epub 2016 Sep 8. Clin Microbiol Infect. 2017. PMID: 27615723 Free PMC article.
-
Genetics: TLR4 promoter variants influence response to UTI.Nat Rev Urol. 2010 Jul;7(7):365. doi: 10.1038/nrurol.2010.94. Nat Rev Urol. 2010. PMID: 20665938 No abstract available.
References
-
- Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085. - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. - PubMed
-
- Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–725. - PubMed
-
- Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008;29:182–191. - PubMed
-
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

