Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy
- PMID: 20505827
- PMCID: PMC2874011
- DOI: 10.1371/journal.pone.0010763
Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy
Abstract
Background: Duchenne muscular dystrophy (DMD) is the most common, lethal disease of childhood. One of 3500 new-born males suffers from this universally-lethal disease. Other than the use of corticosteroids, little is available to affect the relentless progress of the disease, leading many families to use dietary supplements in hopes of reducing the progression or severity of muscle wasting. Arginine is commonly used as a dietary supplement and its use has been reported to have beneficial effects following short-term administration to mdx mice, a genetic model of DMD. However, the long-term effects of arginine supplementation are unknown. This lack of knowledge about the long-term effects of increased arginine metabolism is important because elevated arginine metabolism can increase tissue fibrosis, and increased fibrosis of skeletal muscles and the heart is an important and potentially life-threatening feature of DMD.
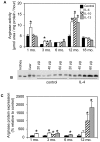
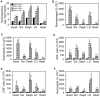
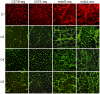
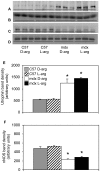
Methodology: We use both genetic and nutritional manipulations to test whether changes in arginase metabolism promote fibrosis and increase pathology in mdx mice. Our findings show that fibrotic lesions in mdx muscle are enriched with arginase-2-expressing macrophages and that muscle macrophages stimulated with cytokines that activate the M2 phenotype show elevated arginase activity and expression. We generated a line of arginase-2-null mutant mdx mice and found that the mutation reduced fibrosis in muscles of 18-month-old mdx mice, and reduced kyphosis that is attributable to muscle fibrosis. We also observed that dietary supplementation with arginine for 17-months increased mdx muscle fibrosis. In contrast, arginine-2 mutation did not reduce cardiac fibrosis or affect cardiac function assessed by echocardiography, although 17-months of dietary supplementation with arginine increased cardiac fibrosis. Long-term arginine treatments did not decrease matrix metalloproteinase-2 or -9 or increase the expression of utrophin, which have been reported as beneficial effects of short-term treatments.
Conclusions/significance: Our findings demonstrate that arginine metabolism by arginase promotes fibrosis of muscle in muscular dystrophy and contributes to kyphosis. Our findings also show that long-term, dietary supplementation with arginine exacerbates fibrosis of dystrophic heart and muscles. Thus, commonly-practiced dietary supplementation with arginine by DMD patients has potential risk for increasing pathology when performed for long periods, despite reports of benefits acquired with short-term supplementation.
Conflict of interest statement
Figures





References
-
- Ishizaki M, Suga T, Kimura E, Shiota T, Kawamo R, et al. Mdx respiratory impairment following fibrosis of the diaphragm. Neuromuscl Disord. 2008;18:342–348. - PubMed
-
- Inkley SR, Oldenburg FC, Vignos PJ. Pulmonary function in Duchenne muscular dystrophy related to stage of disease. Am J Med. 1974;56:297–306. - PubMed
-
- Moriuchi T, Kagawa N, Mukoyama M, Hizawa K. Autopsy analysis of the muscular dystrophies. Tokushima J Exp Med. 1993;40:83–93. - PubMed
-
- Rideau Y, Jankowski LW, Grellet J. Respiratory function in the muscular dystrophies. Muscle Nerve. 1981;4:155–164. - PubMed
-
- Laws N, Hoey A. Progression of kyphosis in mdx mice. J Appl Physiol. 2004;97:1970–1977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

