Osteoblast lineage cells expressing high levels of Runx2 enhance hematopoietic progenitor cell proliferation and function
- PMID: 20506198
- PMCID: PMC3050482
- DOI: 10.1002/jcb.22694
Osteoblast lineage cells expressing high levels of Runx2 enhance hematopoietic progenitor cell proliferation and function
Abstract
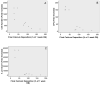
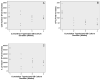
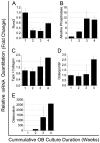
Although osteoblasts (OB) play a key role in the hematopoietic stem cell (HSC) niche, little is known as to which specific OB lineage cells are critical for the enhancement of stem and progenitor cell function. Unlike hematopoietic cells, OB cell surface phenotypic definitions are not well developed. Therefore, to determine which OB lineage cells are most important for hematopoietic progenitor cell (HPC) function, we characterized OB differentiation by gene expression and OB function, and determined whether associations existed between OB and HPC properties. OB were harvested from murine calvariae, used immediately (fresh OB) or cultured for 1, 2, or 3 weeks prior to their co-culture with Lin(-)Sca1(+)c-kit(+) (LSK) cells for 1 week. OB gene expression, alkaline phosphatase activity, calcium deposition, hematopoietic cell number fold increase, CFU fold increase, and fold increase of Lin(-)Sca1(+) cells were determined. As expected, HPC properties were enhanced when LSK cells were cultured with OB compared to being cultured alone. Initial alkaline phosphatase and calcium deposition levels were significantly and inversely associated with an increase in the number of LSK progeny. Final calcium deposition levels and OB culture duration were inversely associated with all HPC parameters, while Runx2 levels were positively associated with all HPC properties. Since calcium deposition is associated with OB maturation and high levels of Runx2 are associated with less mature OB lineage cells, these results suggest that less mature OB better promote HPC proliferation and function than do more mature OB.
© 2010 Wiley-Liss, Inc.
Figures

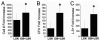


References
-
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. - PubMed
-
- Arai F, Nakamura Y, Gomei Y, Suda T. Characterization of the niche complex molecules in bone marrow. Exp Hematol. 2008;36:S25.
-
- Brooke G, Tong H, Levesque JP, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17:929–940. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

