Characterization of aryl hydrocarbon receptor interacting protein (AIP) mutations in familial isolated pituitary adenoma families
- PMID: 20506337
- PMCID: PMC3065644
- DOI: 10.1002/humu.21292
Characterization of aryl hydrocarbon receptor interacting protein (AIP) mutations in familial isolated pituitary adenoma families
Abstract
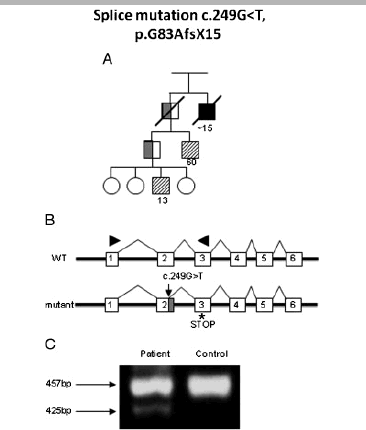
Familial isolated pituitary adenoma (FIPA) is an autosomal dominant condition with variable genetic background and incomplete penetrance. Germline mutations of the aryl hydrocarbon receptor interacting protein (AIP) gene have been reported in 15-40% of FIPA patients. Limited data are available on the functional consequences of the mutations or regarding the regulation of the AIP gene. We describe a large cohort of FIPA families and characterize missense and silent mutations using minigene constructs, luciferase and beta-galactosidase assays, as well as in silico predictions. Patients with AIP mutations had a lower mean age at diagnosis (23.6+/-11.2 years) than AIP mutation-negative patients (40.4+/-14.5 years). A promoter mutation showed reduced in vitro activity corresponding to lower mRNA expression in patient samples. Stimulation of the protein kinase A-pathway positively regulates the AIP promoter. Silent mutations led to abnormal splicing resulting in truncated protein or reduced AIP expression. A two-hybrid assay of protein-protein interaction of all missense variants showed variable disruption of AIP-phosphodiesterase-4A5 binding. In summary, exonic, promoter, splice-site, and large deletion mutations in AIP are implicated in 31% of families in our FIPA cohort. Functional characterization of AIP changes is important to identify the functional impact of gene sequence variants.
Figures
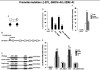

References
-
- Beckers A, Daly AF. The clinical, pathological, and genetic features of familial isolated pituitary adenomas. Eur J Endocrinol. 2007;157:371–382. - PubMed
-
- Beckers A, Vanbellinghen JF, Boikos S, Martari M, Verma S, Daly AF, Raygada M, Keil M, Papademetriou J, Drori-Herishanu L, Horvath A, Nesterova M, Tichomirowa MA, Bours V, Marx S, Agarwal SK, Salvatori R, Stratakis CA. 2008. Germline AIP, MEN1, PRKAR1A, CDKN1B (p27Kip1) and CDKN2C (p18INK4c) gene mutations in a large cohort of pediatric patients with pituitary adenomas occurring in isolation or with associated syndromic features. Proc of the 90th Annual Meet of the Endocrine Soc; OR38-1.
-
- Bertherat J, Chanson P, Montminy M. The cyclic adenosine 3′,5′-monophosphate-responsive factor CREB is constitutively activated in human somatotroph adenomas. Mol Endocrinol. 1995;9:777–783. - PubMed
-
- Bolger GB, Peden AH, Steele MR, MacKenzie C, McEwan DG, Wallace DA, Huston E, Baillie GS, Houslay MD. Attenuation of the activity of the cAMP-specific phosphodiesterase PDE > A5 by interaction with the immunophilin XAP2. J Biol Chem. 2003;278:33351–33363. - PubMed
-
- Buchbinder S, Bierhaus A, Zorn M, Nawroth PP, Humpert P, Schilling T. Aryl hydrocarbon receptor interacting protein gene (AIP) mutations are rare in patients with hormone secreting or non-secreting pituitary adenomas. Exp Clin Endorcrinol Diabetes. 2008;116:625–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

