Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus
- PMID: 20506472
- PMCID: PMC2879086
- DOI: 10.1002/cne.22360
Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus
Abstract
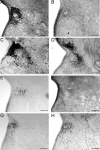
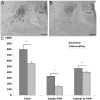
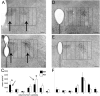
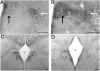
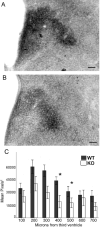
The development of the hypothalamic paraventricular nucleus (PVN) involves several factors that work together to establish a cell group that regulates neuroendocrine functions and behaviors. Several molecular markers were noted within the developing PVN, including estrogen receptors (ER), neuronal nitric oxide synthase (nNOS), and brain-derived neurotrophic factor (BDNF). By contrast, immunoreactive gamma-aminobutyric acid (GABA) was found in cells and fibers surrounding the PVN. Two animal models were used to test the hypothesis that GABA works through GABA(A) and GABA(B) receptors to influence the development of the PVN. Treatment with bicuculline to decrease GABA(A) receptor signaling from embryonic day (E) 10 to E17 resulted in fewer cells containing immunoreactive (ir) ERalpha in the region of the PVN vs. control. GABA(B)R1 receptor subunit knockout mice were used to examine the PVN at P0 without GABA(B) signaling. In female but not male GABA(B)R1 subunit knockout mice, the positions of cells containing ir ERalpha shifted from medial to lateral compared with wild-type controls, whereas the total number of ir ERalpha-containing cells was unchanged. In E17 knockout mice, ir nNOS cells and fibers were spread over a greater area. There was also a significant decrease in ir BDNF in the knockout mice in a region-dependent manner. Changes in cell position and protein expression subsequent to disruption of GABA signaling may be due, in part, to changes in nNOS and BDNF signaling. Based on the current study, the PVN can be added as another site where GABA exerts morphogenetic actions in development.
Figures



Similar articles
-
Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA.Neuroscience. 2003;118(3):585-601. doi: 10.1016/s0306-4522(03)00042-3. Neuroscience. 2003. PMID: 12710969
-
Embryonic GABA(B) receptor blockade alters cell migration, adult hypothalamic structure, and anxiety- and depression-like behaviors sex specifically in mice.PLoS One. 2014 Aug 27;9(8):e106015. doi: 10.1371/journal.pone.0106015. eCollection 2014. PLoS One. 2014. PMID: 25162235 Free PMC article.
-
The vasculature within the paraventricular nucleus of the hypothalamus in mice varies as a function of development, subnuclear location, and GABA signaling.Horm Metab Res. 2012 Jul;44(8):619-24. doi: 10.1055/s-0032-1304624. Epub 2012 Apr 5. Horm Metab Res. 2012. PMID: 22488519
-
gamma-Aminobutyric acid (GABA)--A function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension.Hypertension. 2001 Feb;37(2 Pt 2):614-8. doi: 10.1161/01.hyp.37.2.614. Hypertension. 2001. PMID: 11230344
-
BDNF shifts excitatory-inhibitory balance in the paraventricular nucleus of the hypothalamus to elevate blood pressure.J Neurophysiol. 2021 Oct 1;126(4):1209-1220. doi: 10.1152/jn.00247.2021. Epub 2021 Aug 18. J Neurophysiol. 2021. PMID: 34406887 Free PMC article.
Cited by
-
Sex differences, hormones, and fMRI stress response circuitry deficits in psychoses.Psychiatry Res. 2015 Jun 30;232(3):226-36. doi: 10.1016/j.pscychresns.2015.03.006. Epub 2015 Mar 31. Psychiatry Res. 2015. PMID: 25914141 Free PMC article.
-
BDNF and the central control of feeding: accidental bystander or essential player?Trends Neurosci. 2013 Feb;36(2):83-90. doi: 10.1016/j.tins.2012.12.009. Epub 2013 Jan 18. Trends Neurosci. 2013. PMID: 23333344 Free PMC article. Review.
-
Sex-Dependent Shared and Nonshared Genetic Architecture Across Mood and Psychotic Disorders.Biol Psychiatry. 2022 Jan 1;91(1):102-117. doi: 10.1016/j.biopsych.2021.02.972. Epub 2021 Mar 23. Biol Psychiatry. 2022. PMID: 34099189 Free PMC article.
-
The Notch effector gene Hes1 regulates migration of hypothalamic neurons, neuropeptide content and axon targeting to the pituitary.Dev Biol. 2011 May 1;353(1):61-71. doi: 10.1016/j.ydbio.2011.02.018. Epub 2011 Feb 23. Dev Biol. 2011. PMID: 21354131 Free PMC article.
-
Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease.Front Neuroendocrinol. 2014 Jan;35(1):140-58. doi: 10.1016/j.yfrne.2013.12.001. Epub 2013 Dec 16. Front Neuroendocrinol. 2014. PMID: 24355523 Free PMC article. Review.
References
-
- Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10(4):345–352. - PubMed
-
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5(11):1931–1958. - PubMed
-
- Azumaya Y, Tsutsui K. Localization of galanin and its binding sites in the quail brain. Brain Res. 1996;727(1-2):187–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

