Identification of cerebellin2 in chick and its preferential expression by subsets of developing sensory neurons and their targets in the dorsal horn
- PMID: 20506477
- PMCID: PMC2880495
- DOI: 10.1002/cne.22366
Identification of cerebellin2 in chick and its preferential expression by subsets of developing sensory neurons and their targets in the dorsal horn
Abstract
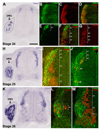
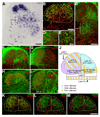
The cerebellins are a family of four secreted proteins, two of which, Cbln1 and Cbln3, play an important role in the formation and maintenance of parallel fiber-Purkinje cell synapses. We have identified the chicken homologue of Cbln2 and, through the use of in situ hybridization, shown that it is expressed by specific subsets of neurons in the dorsal root ganglia (DRGs) and spinal cord starting shortly after those neurons are generated. In the developing spinal cord, Cbln2 is highly expressed by dI1, dI3, dI5, and dILB dorsal interneurons and to a lesser extent by dI2, dI4, dI6, and dILA dorsal interneurons, but not by ventral (v0-v3) interneurons. After the spinal cord has matured and neurons have migrated to their final destinations, Cbln2 is abundant in the dorsal horn. In the DRGs, Cbln2 is expressed by TrkB+ and TrkC+ sensory neurons, but not by TrkA+ sensory neurons. Interestingly, regions of the spinal cord where TrkB+ and TrkC+ afferents terminate (i.e., laminae II, III, IV, and VI) exhibit the highest levels of Cbln2 expression. Cbln2 is also expressed by preganglionic sympathetic neurons and their targets in the sympathetic chain ganglia. Thus, the results show that Cbln2 is frequently expressed by synaptically connected neuronal populations. This, in turn, raises the possibility that if Cbln2, like Cbln1, plays a role in the formation and maintenance of synapses, it may somehow mediate bi-directional communication between discrete populations of neurons and their appropriate neuronal targets.
Figures





Similar articles
-
Localization of cerebellin-2 in late embryonic chicken brain: implications for a role in synapse formation and for brain evolution.J Comp Neurol. 2011 Aug 1;519(11):2225-51. doi: 10.1002/cne.22626. J Comp Neurol. 2011. PMID: 21456003 Free PMC article.
-
SEMA3A regulates developing sensory projections in the chicken spinal cord.J Neurobiol. 2000 Dec;45(4):227-36. J Neurobiol. 2000. PMID: 11077427
-
GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia.J Comp Neurol. 2001 Nov 12;440(2):204-17. doi: 10.1002/cne.1380. J Comp Neurol. 2001. PMID: 11745618
-
Modulation of sensory input to the spinal cord by presynaptic ionotropic glutamate receptors.Arch Ital Biol. 2005 May;143(2):103-12. Arch Ital Biol. 2005. PMID: 16106991 Review.
-
Specification of dorsal spinal cord interneurons.Curr Opin Neurobiol. 2003 Feb;13(1):42-9. doi: 10.1016/s0959-4388(03)00010-2. Curr Opin Neurobiol. 2003. PMID: 12593981 Review.
Cited by
-
Parcellation of cerebellins 1, 2, and 4 among different subpopulations of dorsal horn neurons in mouse spinal cord.J Comp Neurol. 2014 Feb 1;522(2):479-97. doi: 10.1002/cne.23422. J Comp Neurol. 2014. PMID: 23853053 Free PMC article.
-
Cbln1 regulates axon growth and guidance in multiple neural regions.PLoS Biol. 2022 Nov 17;20(11):e3001853. doi: 10.1371/journal.pbio.3001853. eCollection 2022 Nov. PLoS Biol. 2022. PMID: 36395107 Free PMC article.
-
Neuronal aromatase expression in pain processing regions of the medullary and spinal cord dorsal horn.J Comp Neurol. 2017 Nov 1;525(16):3414-3428. doi: 10.1002/cne.24269. Epub 2017 Jul 24. J Comp Neurol. 2017. PMID: 28649695 Free PMC article.
-
Localization of cerebellin-2 in late embryonic chicken brain: implications for a role in synapse formation and for brain evolution.J Comp Neurol. 2011 Aug 1;519(11):2225-51. doi: 10.1002/cne.22626. J Comp Neurol. 2011. PMID: 21456003 Free PMC article.
-
Intravenous recombinant cerebellin 1 treatment restores signalling by spinal glutamate delta 1 receptors and mitigates chronic pain.Br J Pharmacol. 2024 May;181(9):1421-1437. doi: 10.1111/bph.16296. Epub 2024 Jan 24. Br J Pharmacol. 2024. PMID: 38044332 Free PMC article.
References
-
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require Neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. - PubMed
-
- Bao D, Pang Z, Morgan JI. The structure and proteolytic processing of Cbln1 complexes. J Neurochem. 2005;95:618–629. - PubMed
-
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on MATH1. Neuron. 2001;30:411–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

