DE-loop mutations affect beta2 microglobulin stability, oligomerization, and the low-pH unfolded form
- PMID: 20506535
- PMCID: PMC2974830
- DOI: 10.1002/pro.419
DE-loop mutations affect beta2 microglobulin stability, oligomerization, and the low-pH unfolded form
Abstract
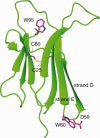
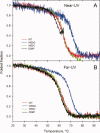
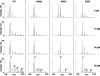
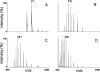
Beta2 microglobulin (beta2m) is the light chain of class-I major histocompatibility complex (MHC-I). Its accumulation in the blood of patients affected by kidney failure leads to amyloid deposition around skeletal joints and bones, a severe condition known as Dialysis Related Amyloidosis (DRA). In an effort to dissect the structural determinants of beta2m aggregation, several beta2m mutants have been previously studied. Among these, three single-residue mutations in the loop connecting strands D and E (W60G, W60V, D59P) have been shown to affect beta2m amyloidogenic properties, and are here considered. To investigate the biochemical and biophysical properties of wild-type (w.t.) beta2m and the three mutants, we explored thermal unfolding by Trp fluorescence and circular dichroism (CD). The W60G mutant reveals a pronounced increase in conformational stability. Protein oligomerization and reduction kinetics were investigated by electrospray-ionization mass spectrometry (ESI-MS). All the mutations analyzed here reduce the protein propensity to form soluble oligomers, suggesting a role for the DE-loop in intermolecular interactions. A partially folded intermediate, which may be involved in protein aggregation induced by acids, accumulates for all the tested proteins at pH 2.5 under oxidizing conditions. Moreover, the kinetics of disulfide reduction reveals specific differences among the tested mutants. Thus, beta2m DE-loop mutations display long-range effects, affecting stability and structural properties of the native protein and its low-pH intermediate. The evidence presented here hints to a crucial role played by the DE-loop in determining the overall properties of native and partially folded beta2m.
Figures
References
-
- Floege J, Ehlerding G. Beta-2-microglobulin-associated amyloidosis. Nephron. 1996;72:9–26. - PubMed
-
- Menaa C, Esser E, Sprague SM. Beta2-microglobulin stimulates osteoclast formation. Kidney Int. 2008;73:1275–1281. - PubMed
-
- Drueke TB. Beta2-microglobulin and amyloidosis. Nephrol Dial Transplant. 2000;15(Suppl 1):17–24. - PubMed
-
- Gejyo F, Yamada T, Odani S, Nakagawa Y, Arakawa M, Kunitomo T, Kataoka H, Suzuki M, Hirasawa Y, Shirahama T, Cohen AS, Schmid K. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

