Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the U.S. Military HIV Natural History Study
- PMID: 20507622
- PMCID: PMC2894737
- DOI: 10.1186/1742-6405-7-14
Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the U.S. Military HIV Natural History Study
Abstract
Background: To examine the outcomes of highly-active antiretroviral therapy (HAART) for individuals with free access to healthcare, we evaluated 2327 patients in a cohort study composed of military personnel and beneficiaries with HIV infection who initiated HAART from 1996 to the end of 2007.
Methods: Outcomes analyzed were virologic suppression (VS) and failure (VF), CD4 count changes, AIDS and death. VF was defined as never suppressing or having at least one rebound event. Multivariate (MV) analyses stratified by the HAART initiation year (before or after 2000) were performed to identify risk factors associated with these outcomes.
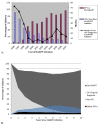
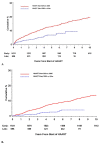
Results: Among patients who started HAART after 2000, 81% had VS at 1 year (N = 1,759), 85% at 5 years (N = 1,061), and 82% at 8 years (N = 735). Five years post-HAART, the median CD4 increase was 247 cells/ml and 34% experienced VF. AIDS and mortality rates at 5 years were 2% and 0.3%, respectively. In a MV model adjusted for known risk factors associated with treatment response, being on active duty (versus retired) at HAART initiation was associated with a decreased risk of AIDS (HR = 0.6, 95% CI 0.4-1.0) and mortality (0.6, 0.3-0.9), an increased probability of CD4 increase ≥ 50% (1.2, 1.0-1.4), but was not significant for VF.
Conclusions: In this observational cohort, VS rates approach those described in clinical trials. Initiating HAART on active duty was associated with even better outcomes. These findings support the notion that free access to healthcare likely improves the response to HAART thereby reducing HIV-related morbidity and mortality.
Figures
References
-
- Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, Montaner JS. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr. 2005;40:288–293. doi: 10.1097/01.qai.0000182847.38098.d1. - DOI - PubMed
-
- Tan R, Westfall AO, Willig JH, Mugavero MJ, Saag MS, Kaslow RA, Kempf MC. Clinical outcome of HIV-infected antiretroviral-naive patients with discordant immunologic and virologic responses to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:553–558. doi: 10.1097/QAI.0b013e31816856c5. - DOI - PubMed
-
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

